Preparation method of hexachlorobutadiene
A technology of hexafluorobutadiene and synthesis method, which is applied in the field of preparation of hexafluorobutadiene, and can solve problems such as separation difficulties, product yield decline, and difficult extraction of perfluorocyclobutene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Preparation of Grignard reagents:
[0022]A) In a 3L round-bottomed three-necked flask with a condenser, add 108g of magnesium strips (4.5mol), a few grains of iodine, and at the same time add about 500ml of anhydrous ether, heat to boiling, and dissolve 4.5mol of bromobenzene in 200ml of ether solution , first drop a few drops, and after the Grignard reaction is triggered, slowly add the remaining bromophenetole solution dropwise to generate a Grignard reagent with a concentration of about 3 mol / l. By increasing the amount of diethyl ether, different concentrations of bromobenzene and magnesium can be obtained such as 1mol / l, 2mol / , etc. to prepare Grignard reagent.
[0023] B) In a 3L round-bottomed three-necked flask with a condenser, add 27.8g of lithium metal (4.0mol) and a few grains of iodine, and at the same time add about 1000ml of tetrahydrofuran. Add a few drops dropwise to initiate the Grignard reaction, then slowly add the remaining bromobenzenetetrahydrof...
Embodiment 1
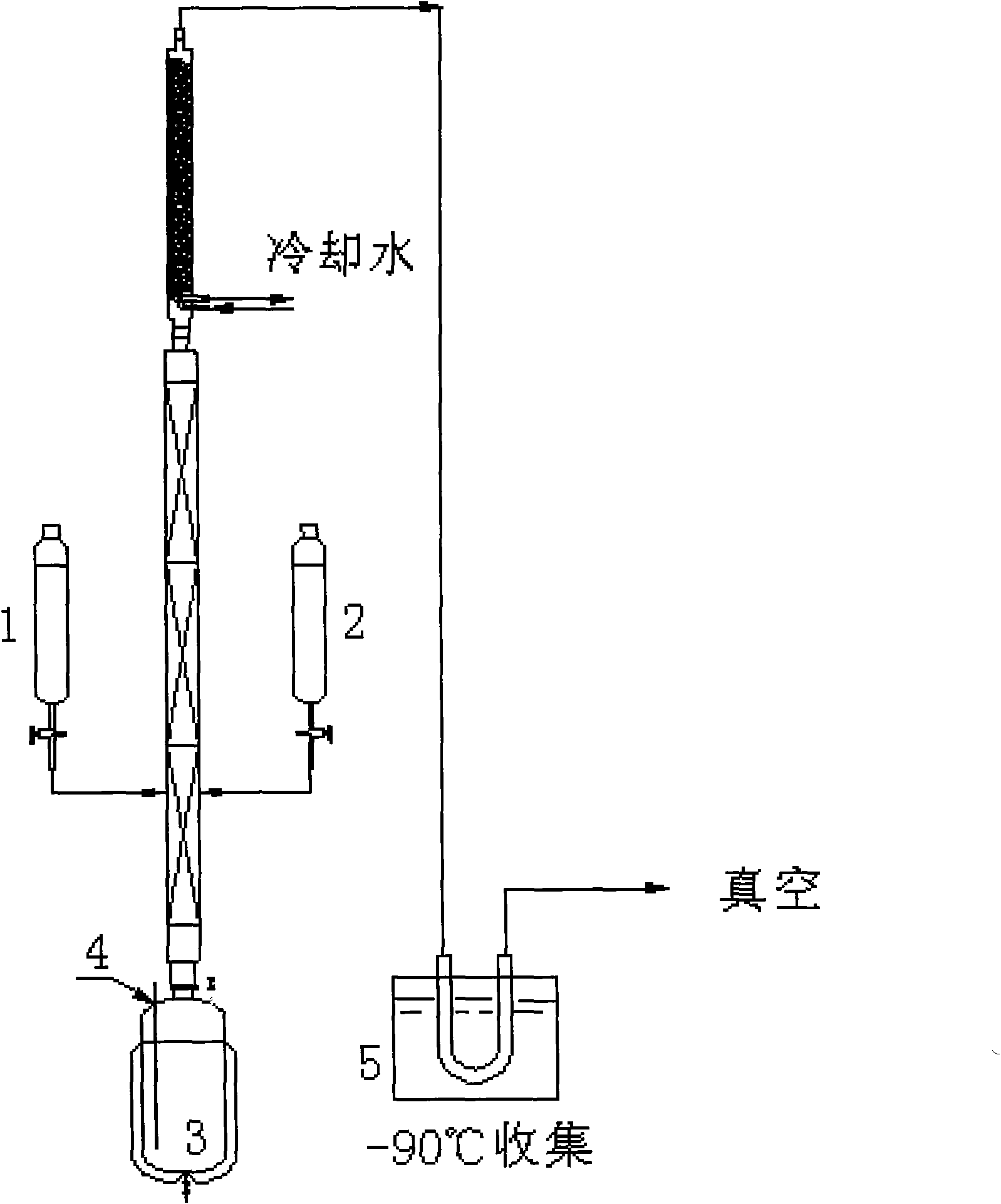
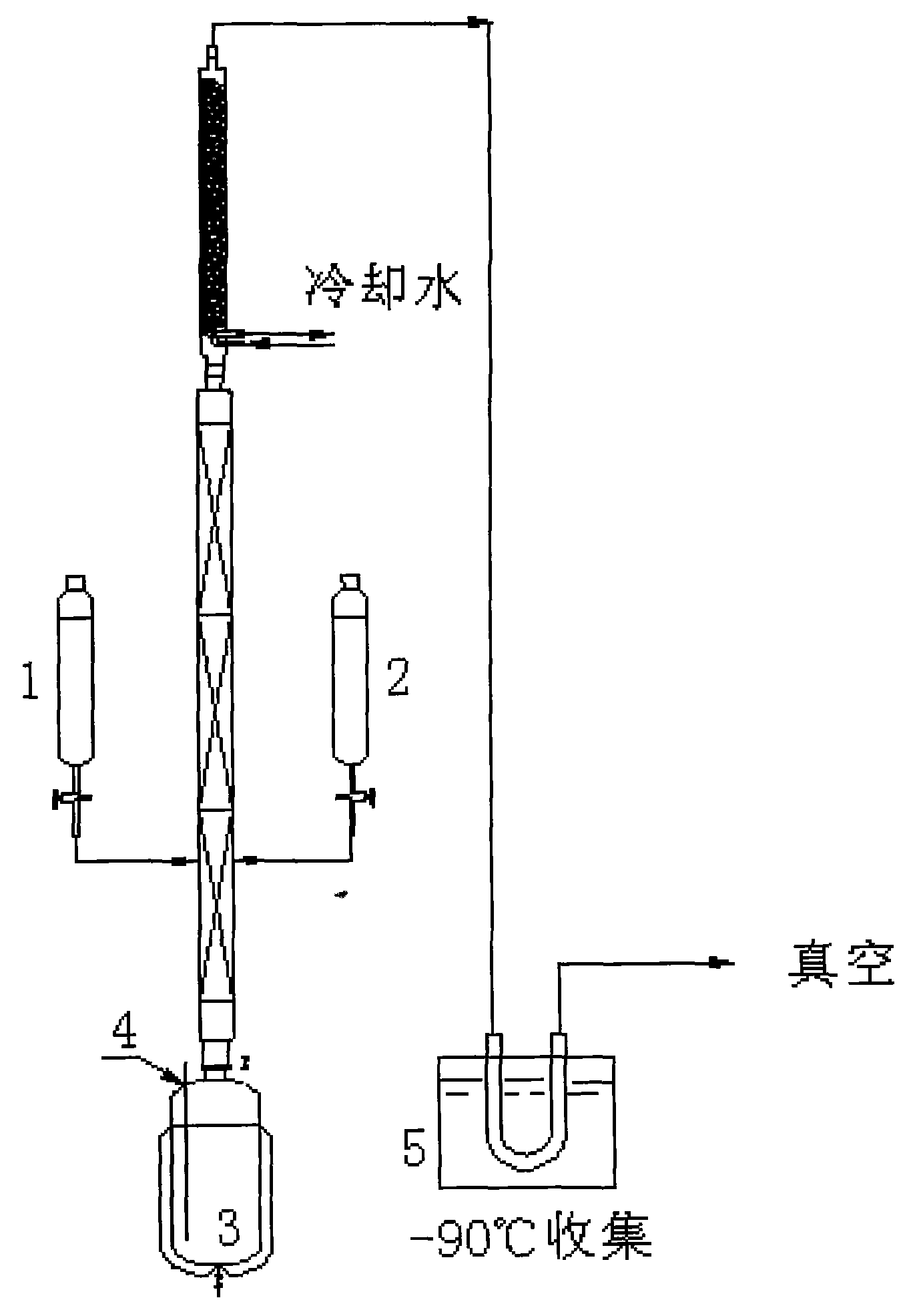
[0026] Such as figure 1 In the airtight device shown, 400ml of tetrahydrofuran as a solvent is pre-added in a 2000ml tower kettle 3 with a thermocouple 4 . Add 1000ml (2.0mol) of the Grignard reagent prepared by the above method A and 500ml of 454g (1.0mol) diiodooctafluorobutane with a concentration of 2mol / l into the two dropping funnels 1 and 2 shown in the figure. For tetrahydrofuran solution, nitrogen gas is used to replace all the gases in the equipment and then vacuumize. The vacuum degree is maintained at 300mmHg. Heat the tower kettle to keep the tetrahydrofuran boiling until it reaches full reflux. Slowly add Grignard reagent and diiodooctafluorobutane solution to the middle of the tower at the same time, control the rate of addition, so that the entire solution is added dropwise in about 3-4 hours, collect the crude product in the product collection device 5, and cool it in the product collection device The medium is -90°C. After the reaction is completed, the crud...
Embodiment 2
[0028] As the equipment described in Example 1, add solvent 400ml tetrahydrofuran in 2000ml tower kettle with thermocouple, add respectively concentration be 3mol / l bromobenzene and Mg in 1,2 two dropping funnels according to above-mentioned A The prepared Grignard reagent 1000ml (3.0mol) and 682g (1.5mol) diiodooctafluorobutane solution in 500ml tetrahydrofuran, after all the air in the device was replaced with nitrogen, vacuumed to maintain a vacuum of 100mmHg, heating tower Kettle, keep tetrahydrofuran boiling to total reflux, control the cooling temperature at the top of the tower at 6-7°C, keep a certain amount of reflux in the tower kettle, after the total reflux, slowly drop Grignard reagent and diiodooctafluorobutane from the middle of the tower at the same time Alkanes solution, control the drop rate, so that the whole solution is added dropwise in about 3-4 hours, collect the crude product in a low-temperature cold trap at -90°C, after the reaction is completed, place...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com