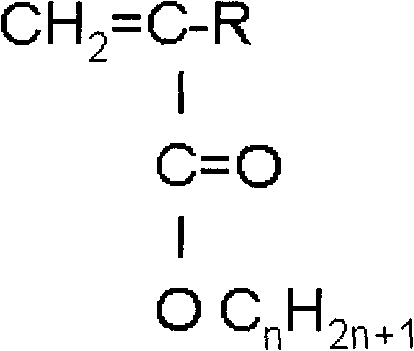A kind of preparation method of polymer phase change material
A phase change material and polymer technology, applied in heat exchange materials, chemical instruments and methods, coatings, etc., can solve problems such as poor heat storage performance of copolymers, and achieve stable performance, good application prospects, and thermal stability. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The preparation method (hereinafter referred to as the preparation method) of the polymer phase change material designed by the present invention, the preparation method adopts the following techniques:
[0013] Using 1 to 5 kinds of n-alkyl (meth)acrylates as raw materials, the general formula of the n-alkyl (meth)acrylates is CH 2 =C(CH 3 )-COOC n h 2n+1 or CH 2 =CH-COOC n h 2n+1 , where n=10-50, when there are 2-5 kinds of n-alkyl (meth)acrylates, the molar content of any one of the raw materials is not less than 10%, and all kinds of (meth)acrylic acid in the mixture The sum of the molar contents of n-alkyl esters is 100%, and the polymerization inhibitor is removed by washing with a conventional sodium hydroxide or potassium hydroxide solution with a mass percentage of 1 to 10%, and after vacuum distillation, one of the following methods is used Polymerization occurs:
[0014] (1) Irradiation-initiated polymerization: said irradiation-initiated refers to the...
Embodiment 1
[0038] Methyl methacrylate is mixed with 1-stearyl alcohol in a molar ratio of 1.1:1, and 0.5 wt% of methyl methacrylate weight (the same below) is added as a catalyst for p-toluenesulfonic acid, 0.1 wt% of polymerization inhibitor agent hydroquinone, reacted at 80°C for 4 hours, steamed out the generated methanol to obtain n-octadecyl methacrylate (OMA), which was washed with deionized water, 10% sodium hydroxide solution alkali and 10 After salt washing with 1% sodium chloride solution, wash again with deionized water to neutrality, dry with anhydrous calcium chloride for 8 hours, distill under reduced pressure, collect 80kPa, 70~100 ℃ fraction, and the yield is 89%.
[0039] with FeCl 2 4H 2 O is the catalyst, triphenylphosphine (PPh 3 ) is a complexing agent, n-ethyl 2-bromopropionate (EBP) is an initiator, toluene is used as a solvent, and the weight ratio of n-octadecyl methacrylate to toluene is 1: 1. / (EBP) / (FeCl 2 4H 2 O) / (PPh 3 ) when the ratio is 100 / 1 / 30 / 2, r...
Embodiment 2
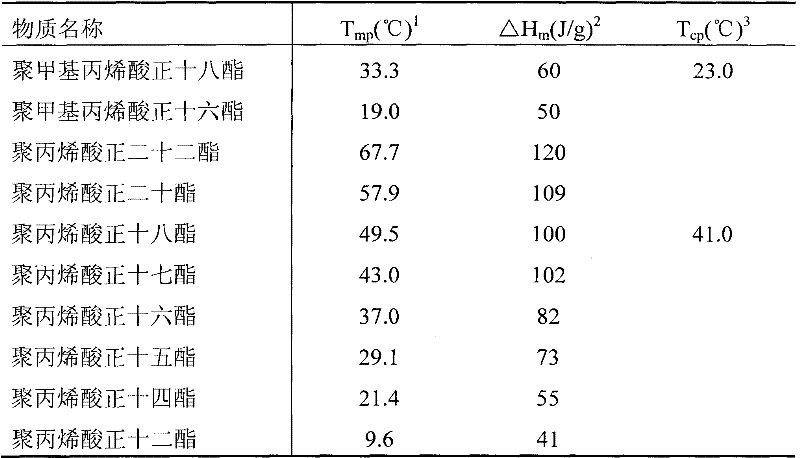
[0047] Replace the 1-stearyl alcohol in Example 1 with 1-Cetyl Alcohol, and other reagents and process parameters are the same as in Example 1 to obtain poly-hexadecyl methacrylate with an endothermic temperature of 19.0° C. (Table 1) , melting enthalpy 50J / g, thermal decomposition temperature in air is 259 ℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| heat release | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com