Method for preparing rare earth oxides
A rare earth oxysulfide, rare earth technology, applied in rare earth metal compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problem that rare earth oxysulfide cannot be directly prepared
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The embodiment of the present invention discloses a preparation method of spherical sulfur oxide, comprising:
[0024] a, after mixing the rare earth salt solution and PVP, dissolve it with an organic solvent to obtain the first reaction solution;
[0025] b. Obtaining the second reaction solution after dissolving thiourea with an organic solvent;
[0026] c. After mixing the first reaction solution and the second reaction solution, heating, and constant temperature reaction to obtain the precursor;
[0027] d. Spherical rare earth sulfur oxides obtained by calcining the precursor in a sulfur-containing atmosphere.
[0028] According to the present invention, the rare earth salt solution is a rare earth nitrate solution and / or a rare earth chloride solution, and the rare earth in the rare earth salt solution is lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), samarium ( Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), er...
Embodiment 1
[0033] 1. Take 40mL of 0.5mol / L Gd(NO 3 ) 3 solution and mix it with 20g of PVP, add the mixed solution into the mixed solution of 150mL ethanol and 100mL ethylene glycol, and stir until the PVP is completely dissolved to obtain the first reaction solution.
[0034] 2. Dissolve 1.1 g of thiourea in a mixed solution of 50 mL of ethanol and 50 mL of ethylene glycol to obtain a second reaction solution.
[0035] 3. Add the second reaction solution dropwise to the first reaction solution, stir evenly, transfer to an autoclave, and react at a constant temperature at 210°C for 24 hours to obtain a reaction product, mix the reaction product with ethanol and transfer to a centrifuge for centrifugation Separated to obtain the precursor.
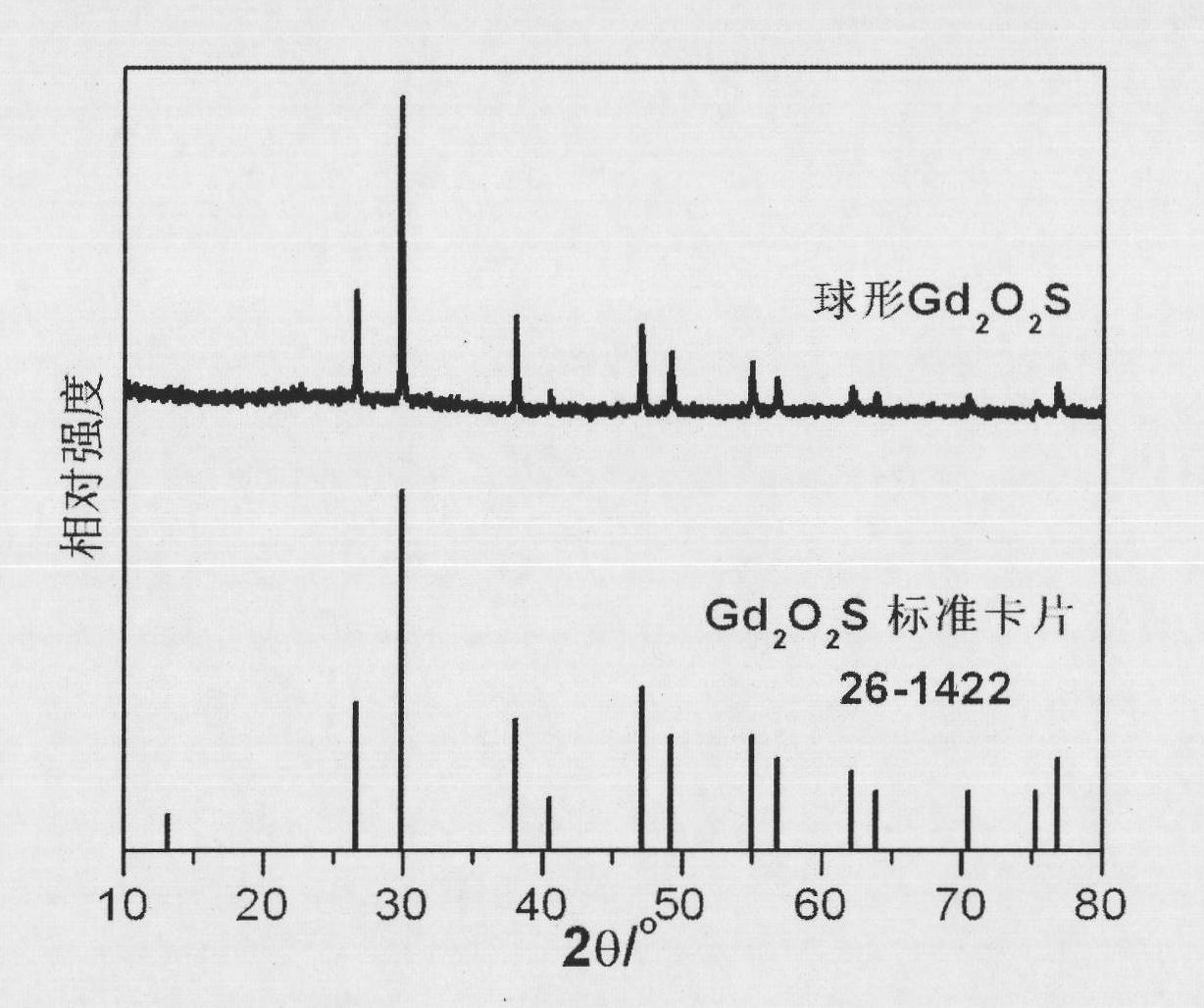
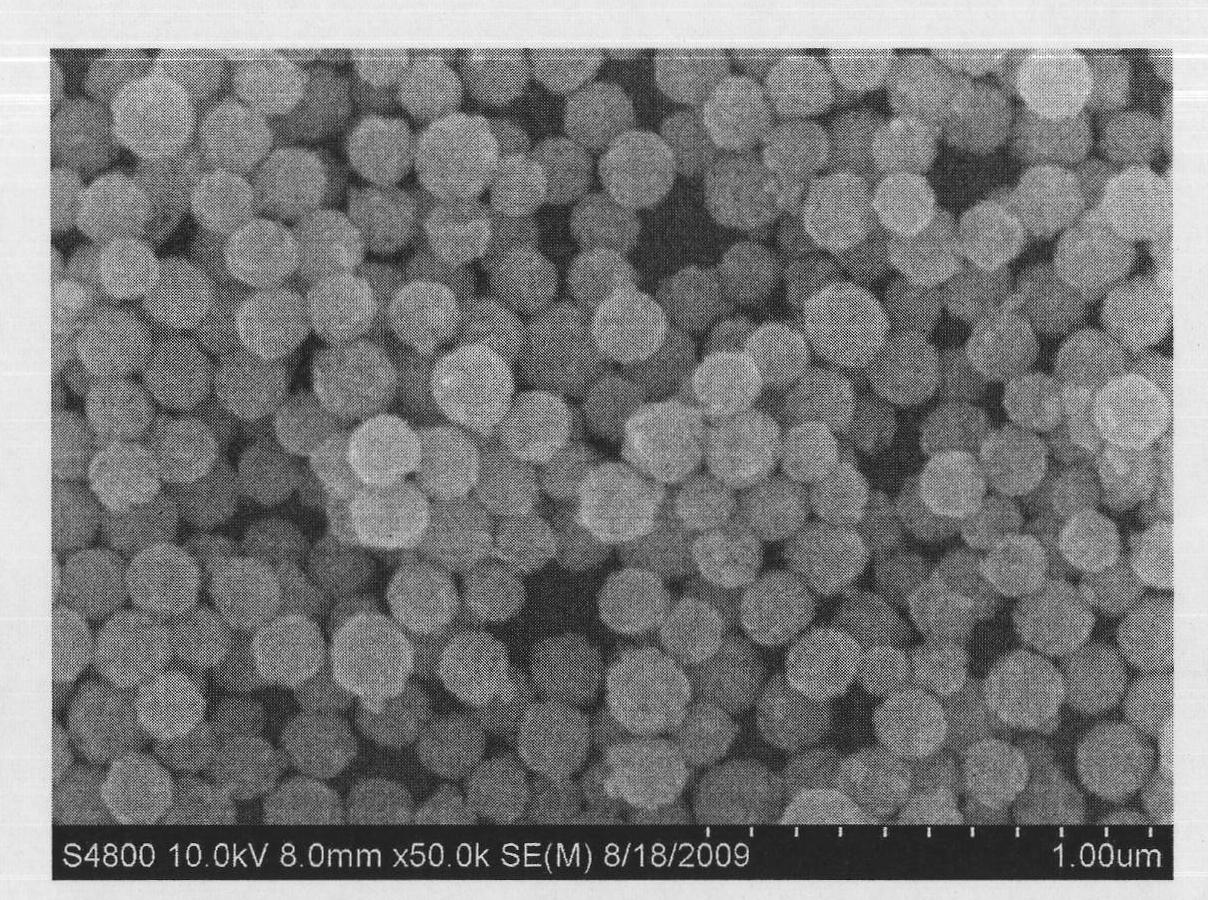
[0036] 4. Put the precursor at 700℃ in N 2 After calcination in / S atmosphere for 2 hours, the calcined product was cooled to room temperature to obtain spherical Gd 2 o 2 S.
[0037] see figure 1 Gd prepared for this example 2 o 2 SX-ray dif...
Embodiment 2
[0039] 1. Take 39mL of 0.5mol / L Gd(NO 3 ) 3 solution, 10mL 0.05mol / L Eu(NO 3 ) 3 solution, the weighed Gd(NO 3 ) 3 solution and Eu(NO 3 ) 3 After the solution was mixed with 20g of PVP, 250mL of ethylene glycol was added, and stirred until all the PVP was dissolved to obtain the first reaction solution.
[0040] 2. Dissolve 1.1 g of thiourea in 100 mL of ethylene glycol to obtain a second reaction solution.
[0041] 3. Add the second reaction solution dropwise to the first reaction solution, stir evenly, transfer to an autoclave, and react at a constant temperature at 200°C for 24 hours to obtain a reaction product, mix the reaction product with ethanol and transfer to a centrifuge for centrifugation Separated to obtain the precursor.
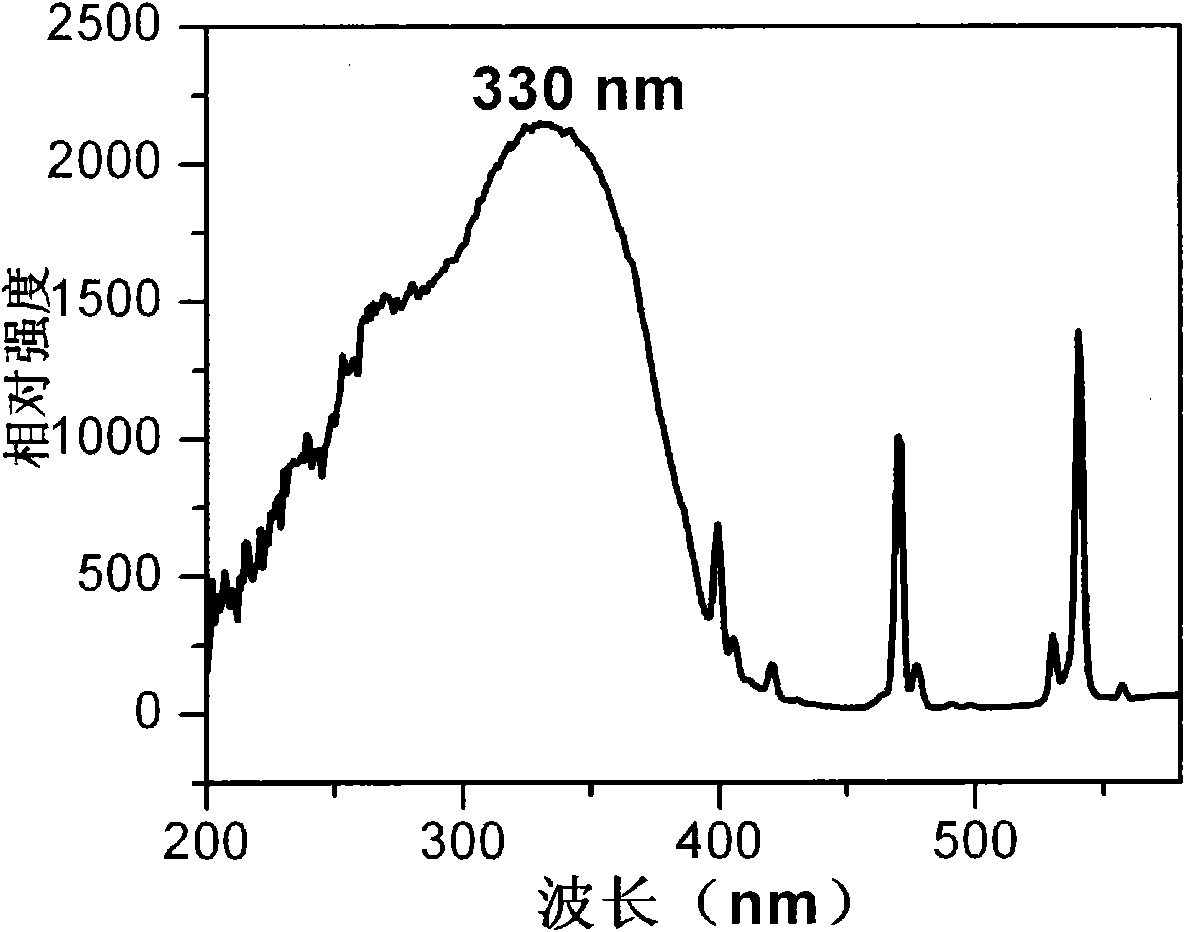
[0042] 4. Put the precursor at 700℃ in ℃S 2 After calcination in the atmosphere for 2 hours, the calcined product was cooled to room temperature to obtain spherical Gd 1.95 Eu 0.05 o 2 S.
[0043] see image 3 Gd prepared for this...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com