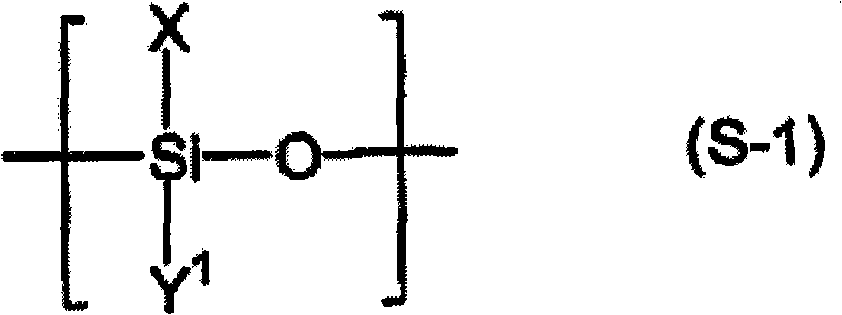
Liquid crystal aligning agent, method for producing liquid crystal alignment film, and liquid crystal display device
A technology of liquid crystal alignment agent and polyorganosiloxane, which is applied in optics, instruments, nonlinear optics, etc., and can solve problems such as large ray irradiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0201] The present invention will be described more specifically by way of examples below, but the present invention is not limited to the examples.
[0202] Hereinafter, the weight average molecular weight of the polyorganosiloxane is a polystyrene-equivalent value measured by gel permeation chromatography under the following conditions.
[0203] The solution viscosity of a polyamic-acid solution is the value measured at 25 degreeC using the E-type viscometer.
[0204] Column: manufactured by Tosoh Corporation, TSK gel GRCXLII
[0205] Solvent: THF
[0206] Temperature: 40°C
[0207] Pressure: 68kgf / cm 2
[0208]
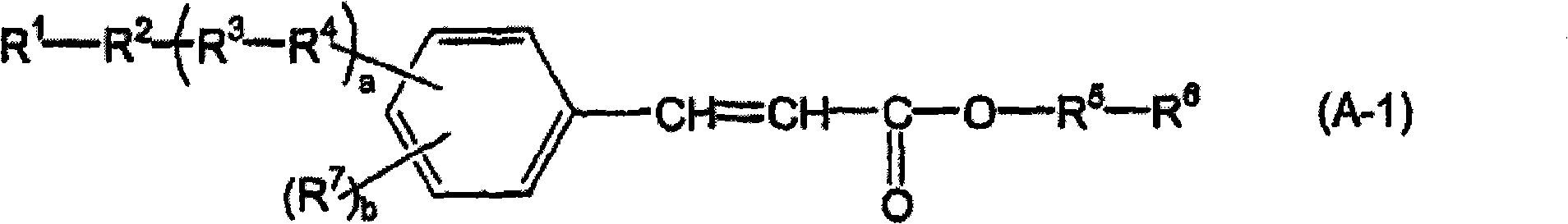
[0209] [Synthesis of the compound represented by the above formula (A-1)]
Synthetic example 1
[0211] Compound (A-1-C4-1) was synthesized according to the following scheme.
[0212]
[0213]Add 53g of 4,4,5,5,6,6,6-heptafluorohexanol and 100mL of pyridine into a 500mL eggplant-shaped bottle, and cool with ice. A solution obtained by dissolving 63 g of p-toluenesulfonyl chloride in 100 mL of pyridine was added dropwise over 30 minutes, and the reaction was further stirred at room temperature for 5 hours. After the reaction was completed, 120 mL of concentrated hydrochloric acid and 500 g of ice were added to the reaction mixture, ethyl acetate was further added, and the organic layer was washed successively with water and saturated aqueous sodium bicarbonate solution. The organic layer was dried over magnesium sulfate, then concentrated and dried to obtain 96 g of compound (A-1-C4-1A).
[0214] Then, in a 500mL three-necked flask with a thermometer, add 96g of the above-mentioned compound (A-1-C4-1A), 55g of methyl 4-hydroxybenzoate, 124g of potassium carbonate and 3...
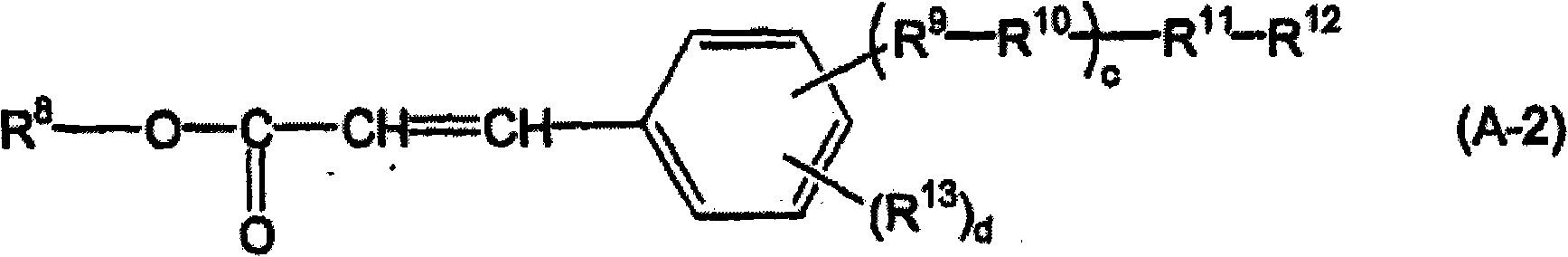
Synthetic example 2
[0219] Compound (A-2-C1-1) was synthesized according to the following scheme.
[0220]
[0221] Add 22g of 4-iodophenol, 16g of hexyl acrylate, 14mL of triethylamine, 2.3g of tetrakis(triphenylphosphine) palladium and 1L of N,N-dimethyl Formamide, dry the system thoroughly. Then the above mixture was heated to 90° C. and stirred for 2 hours under nitrogen flow to react. After the reaction was completed, dilute hydrochloric acid was added and extracted with ethyl acetate. The organic layer was washed with water, dried over magnesium sulfate, concentrated, column purified with a mixed solvent of hexane and ethyl acetate, concentrated and dried to obtain 12 g of compound (A-2-C1-1A).
[0222] Then, add 12g of the above-mentioned compound (A-2-C1-1A), 5.5g of succinic anhydride, and 0.6g of 4-dimethylaminopyridine into a 200mL three-necked flask with a thermometer, a nitrogen gas introduction tube, and a reflux tube, and fully dry the system . Then, 5.6 g of triethylamine a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solution viscosity | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com