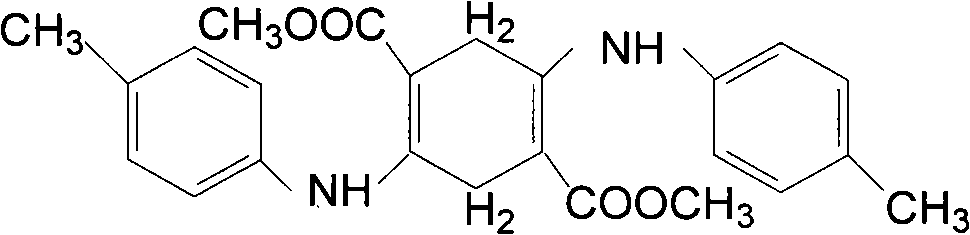
Method for preparing 2,5-diparamethylaniline terephthalic acid (DTTA)
A methylaniline-based, terephthalic acid technology, applied in chemical instruments and methods, cyanide reaction preparation, organic compound preparation and other directions, can solve the problem of hydrolysis, oxidation reaction is difficult to carry out completely, the reaction is difficult, the product purity is low, etc. It can achieve the effect of easy separation, purification and recycling, reasonable and convenient preparation process, and safe and reliable preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
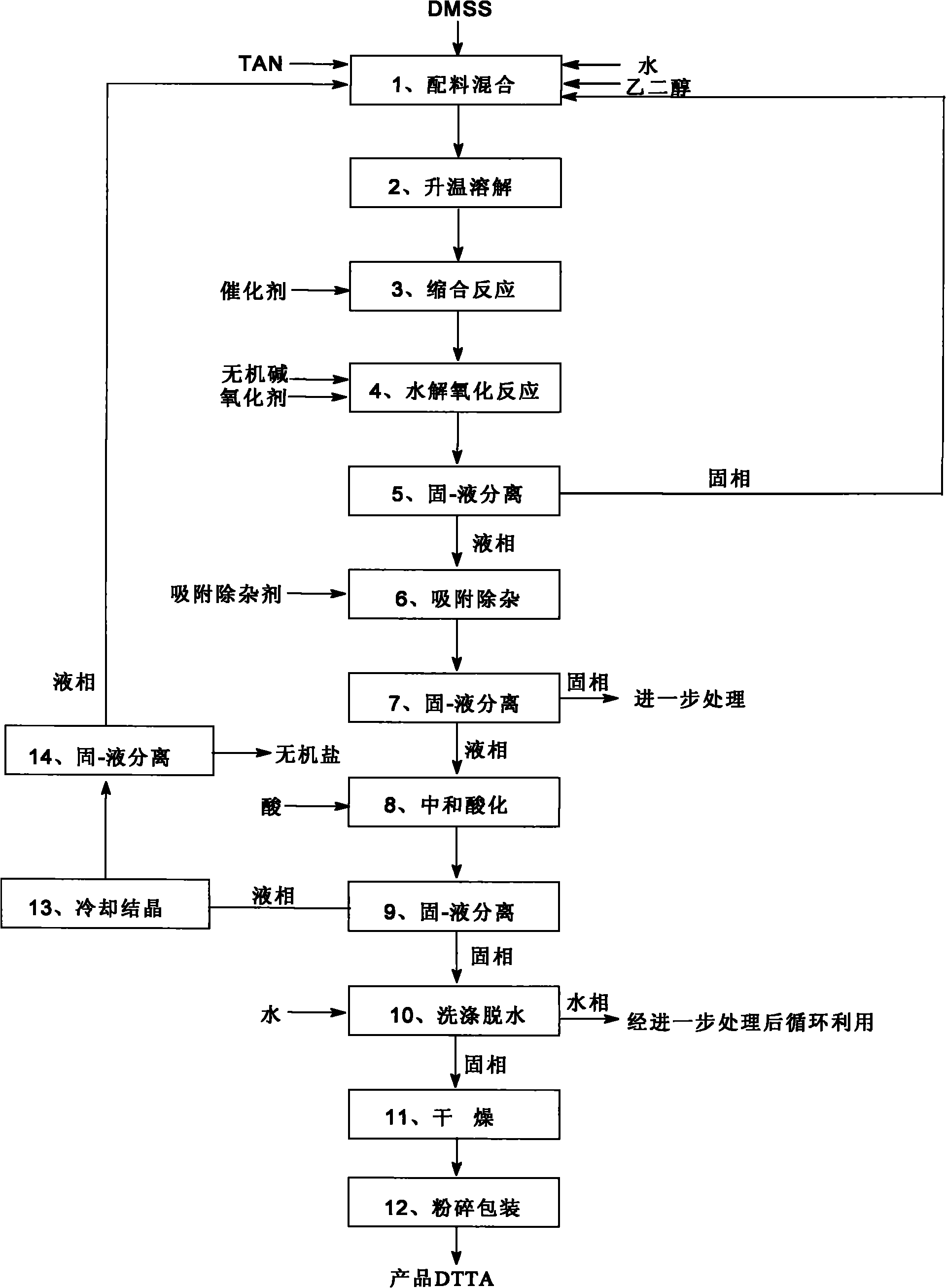
[0057] The main process equipments are: condensation reactor, hydrolysis oxidation reactor, neutralization and acidification reactor, solid-liquid separation equipment and drying equipment, among which the condensation reactor, hydrolysis oxidation reactor and neutralization and acidification reactor are tank-type stirring chemical reactor.
[0058] As shown in the figure, a method for preparing 2,5-di-p-methylanilino terephthalic acid DTTA is to use dimethyl succinate DMSS and p-methylaniline TAN as raw materials through condensation reaction, Hydrolysis oxidation reaction, neutralization and acidification reaction are prepared to obtain 2,5-di-p-methylanilino terephthalic acid, and the method steps are as follows:
[0059] (1) Mixing of ingredients: the equipment tank type stirring chemical reactor used is a glass-lined reactor, and the raw materials 6.0kg dimethyl succinylate DMSS and 5.1kg p-methylaniline TAN and dispersant 54.0kg ethylene glycol Alcohol and 12.0kg water ...
Embodiment 2
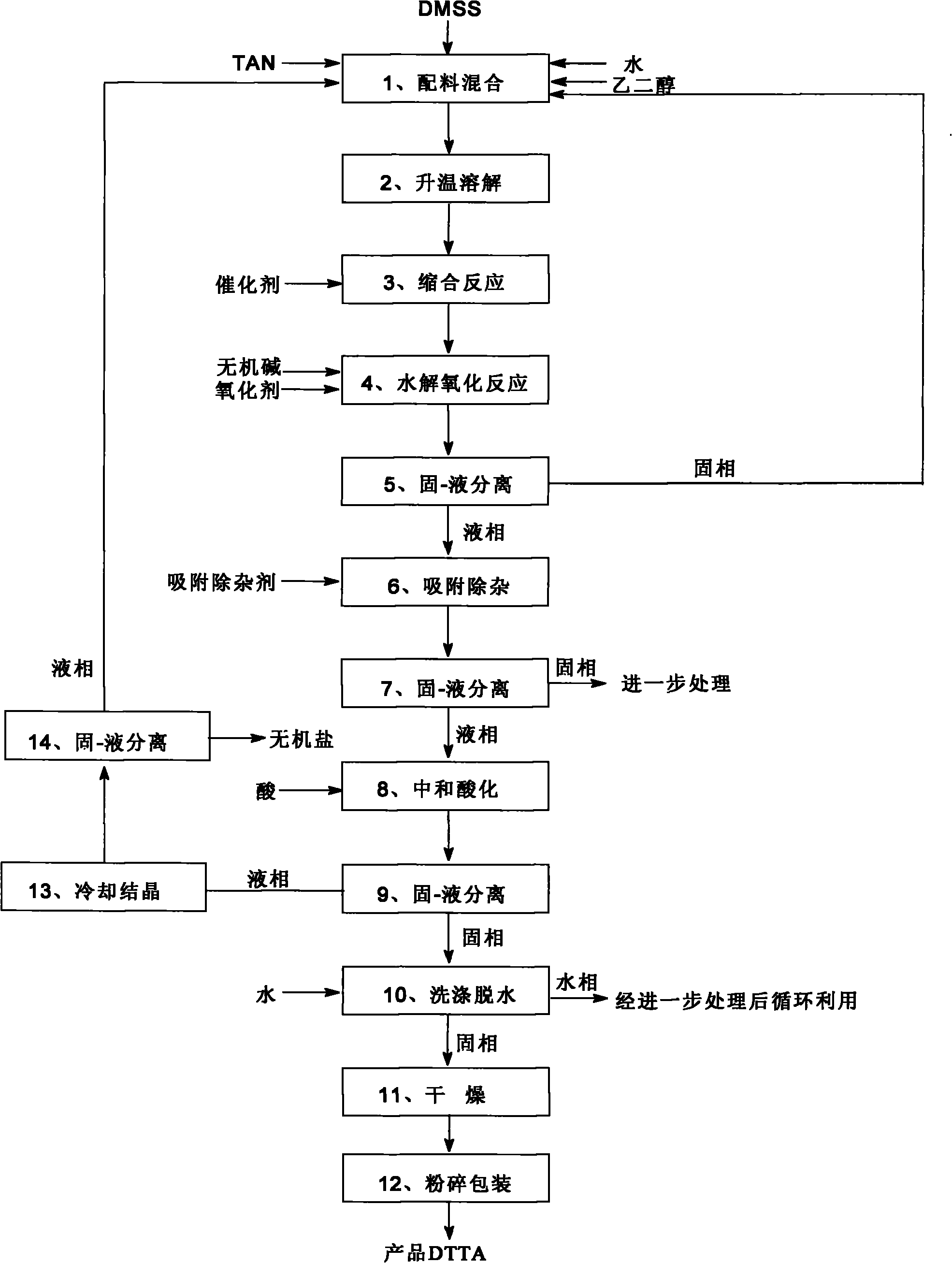
[0072]The main process equipment is: the main process equipment is: condensation reactor, hydrolysis oxidation reactor, neutralization acidification reactor, solid-liquid separation equipment and drying equipment, among which condensation reactor, hydrolysis oxidation reactor, neutralization acidification reactor It is a tubular chemical reactor.
[0073] As shown in the figure, a method for preparing 2,5-di-p-methylanilino terephthalic acid DTTA is to use dimethyl succinate DMSS and p-methylaniline TAN as raw materials through condensation reaction, Hydrolysis oxidation reaction, neutralization and acidification reaction are prepared to obtain 2,5-di-p-methylanilino terephthalic acid, and the method steps are as follows:
[0074] (1) Mixing of ingredients: 6.0kg of dimethyl succinyl succinate DMSS and 5.1kg of p-methylaniline TAN and 54.0kg of dispersant ethylene glycol and 12.0kg of water are added to the tubular chemical reactor. Dimethyl succinyl succinate DMSS: the molar...
Embodiment 3
[0087] The main process equipment is: the main process equipment is: condensation reactor, hydrolysis oxidation reactor, neutralization acidification reactor, solid-liquid separation equipment and drying equipment, among which condensation reactor, hydrolysis oxidation reactor, neutralization acidification reactor for static mixers.
[0088] As shown in the figure, a method for preparing 2,5-di-p-methylanilino terephthalic acid DTTA is to use dimethyl succinate DMSS and p-methylaniline TAN as raw materials through condensation reaction, Hydrolysis oxidation reaction, neutralization and acidification reaction are prepared to obtain 2,5-di-p-methylanilino terephthalic acid, and the method steps are as follows:
[0089] (1) Mixing of ingredients: 6.0kg of dimethyl succinyl succinate DMSS and 5.1kg of p-toluene TAN as raw materials and 54.0kg of ethylene glycol and 12.0kg of dispersant as water are added to the static mixer. Dimethyl succinate DMSS: the mol ratio of p-methylanili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com