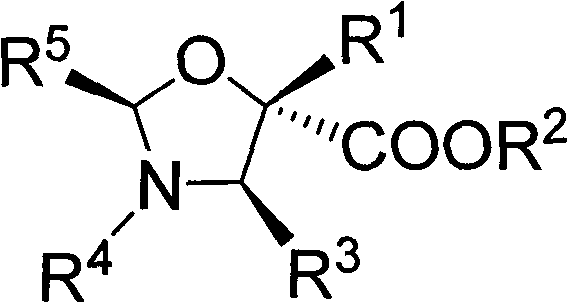
Method for synthesizing polyarylation substituted oxazolidine
A technology of oxazolidine and polyaryl, which is applied in the field of drug synthesis and chemical engineering, can solve the problems of insufficient diversity of product structure, narrow substrate reaction applicability, and low substrate adaptability, etc., and achieve rich and diverse reaction product structures, Broad reactivity and substrate applicability, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] N-benzylidene phenyl imine (0.25mmol), benzaldehyde (0.25mmol), Molecular sieve (0.1g), Lewis acid (Yb(OTf) 3 , 0.025mmol) and Rh 2 (OAc) 4 (0.005mmol) The reaction system consisting of co-catalysts was protected by nitrogen, then dichloromethane (1.0ml) was added, and then cooled to -20°C, and 1.0mL of dichloromethane was mixed with p-methoxyphenyldiazoacetic acid methyl The ester solution (0.275 mmol) was added dropwise to the reaction system within 1 hour using an auto-sampling pump. At the end of the injection, stir at -20°C for 0.5 hours, then add saturated NaHCO dropwise to the reaction system 3 Aqueous solution (0.1 ml) was used to quench the reaction. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:50~1:30) to obtain pure polyaryl-substituted oxazolidine products. Yield 69%, diastereoselectivity greater than 70:30.
Embodiment 2
[0037] N-benzylidene phenyl imine (0.25mmol), benzaldehyde (0.25mmol), Molecular sieve (0.1g), Lewis acid (Yb(OTf) 3 , 0.025mmol) and Rh 2 (OAc) 4 (0.005mmol) reaction system nitrogen protection of co-catalyst composition, add toluene (1.0ml) again, then cool to-20 ℃, under stirring condition, p-methoxyphenyl diazoacetic acid methyl ester (0.275mmol) of 1.0mL toluene ) solution was added dropwise to the reaction system within 1 hour with an autosampler pump. At the end of the injection, stir at -20°C for 0.5 hours, then add saturated NaHCO dropwise to the reaction system 3 Aqueous solution (0.1 ml) was used to quench the reaction. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:50~1:30) to obtain pure polyaryl-substituted oxazolidine products. Yield 63%, diastereoselectivity greater than 70:30.
Embodiment 3
[0039] N-benzylidene phenyl imine (0.25mmol), benzaldehyde (0.25mmol), Molecular sieve (0.1g), Lewis acid (Yb(OTf) 3 , 0.025mmol) and Rh 2 (OAc) 4 (0.005mmol) The reaction system composed of co-catalyst is protected by nitrogen, then p-xylene (1.0ml) is added, then cooled to -20°C, and 1.0mL of p-xylene in p-methoxyphenyldiazoacetic acid methyl The ester (0.275 mmol) solution was added dropwise to the reaction system within 1 hour using an auto-sampling pump. At the end of the injection, stir at -20°C for 0.5 hours, then add saturated NaHCO dropwise to the reaction system 3 Aqueous solution (0.1 ml) was used to quench the reaction. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:50~1:30) to obtain pure polyaryl-substituted oxazolidine products. Yield 57%, diastereoselectivity greater than 70:30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com