Anti-drug resistance antibiotic
A technology of antibiotics and drug resistance, applied in the direction of antibacterial drugs, drug devices, and resistance to vector-borne diseases, etc., to achieve a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
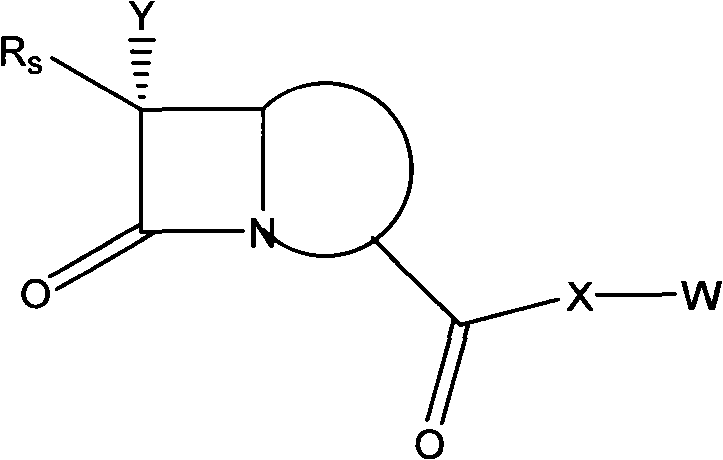
[0036] This embodiment provides one of the preparation methods of the compound in the general formula "Structural Formula 1";
[0037] Preparation of 6-acetophenoxyaminopenicillinic acid-2-diethylaminoethyl ester hydrochloride
[0038] Take 39.1g (0.1mol) of penicillin V and dissolve in 100ml of acetonitrile. Add 39 g (0.15 mol) of 2-diethylaminobromoethane hydrogen bromide, and stir at room temperature for 3 hours. Add 8 g of sodium bicarbonate to the reaction, and stir at room temperature for 2 hours. After evaporating the solvent to dryness, 250ml of ethyl acetate was added, and the mixture was washed with water three times (3×100ml). The organic layer was dried over anhydrous sodium sulfate and the solid was filtered off. 3.5 g of hydrochloric acid was added to the filtrate with stirring. The solid product was collected by filtration. After drying, 38 g of a hygroscopic product was obtained with a yield of 78.2%. Solubility in water: 50mg / ml; Elemental analysis: C 2...
Embodiment 2
[0040] This example provides the results of the drug of the present invention penetrating the blood-brain barrier.
[0041] The rate at which the prodrug penetrates the skin and the blood-brain barrier of live hairless mice was compared. Donor was dissolved in isopropanol by 1ml of 20% prodrug solution (6-(2,6-dimethoxybenzamide) penicillin acid-2-diethylaminoethyl ester hydrochloride solution, 6-(5 -Methyl-3-phenyl-2-isoxazoline-4-carboxamido)penicillinic acid-2-diethylaminoethyl ester hydrochloride solution or 6-[3-(o-chlorophenyl)-5 -Methyl-4-isoxazolecarboxamido]penicillinic acid-2-diethylaminoethyl ester hydrochloride solution) composition. Apply it 10cm on the back of the hairless mouse 2 parts. After 2 hours, the mice were sacrificed. Take 1g of blood, 1g of liver, 1g of kidney, 1g of muscle, and 1g of brain, add 5ml of methanol respectively, mix well with a homogenizer, centrifuge for 5 minutes, and measure by HPLC. The concentration of methicillin in blood is 50+...
Embodiment 3
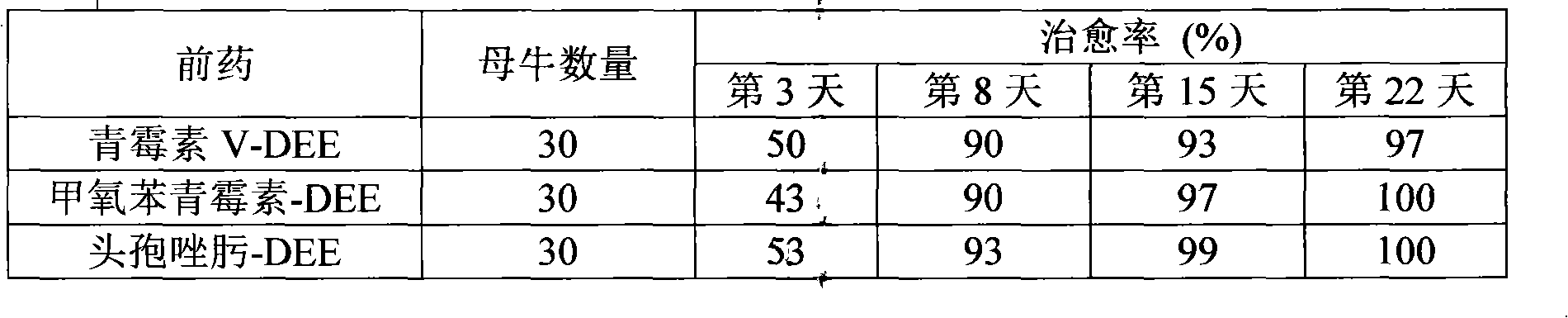
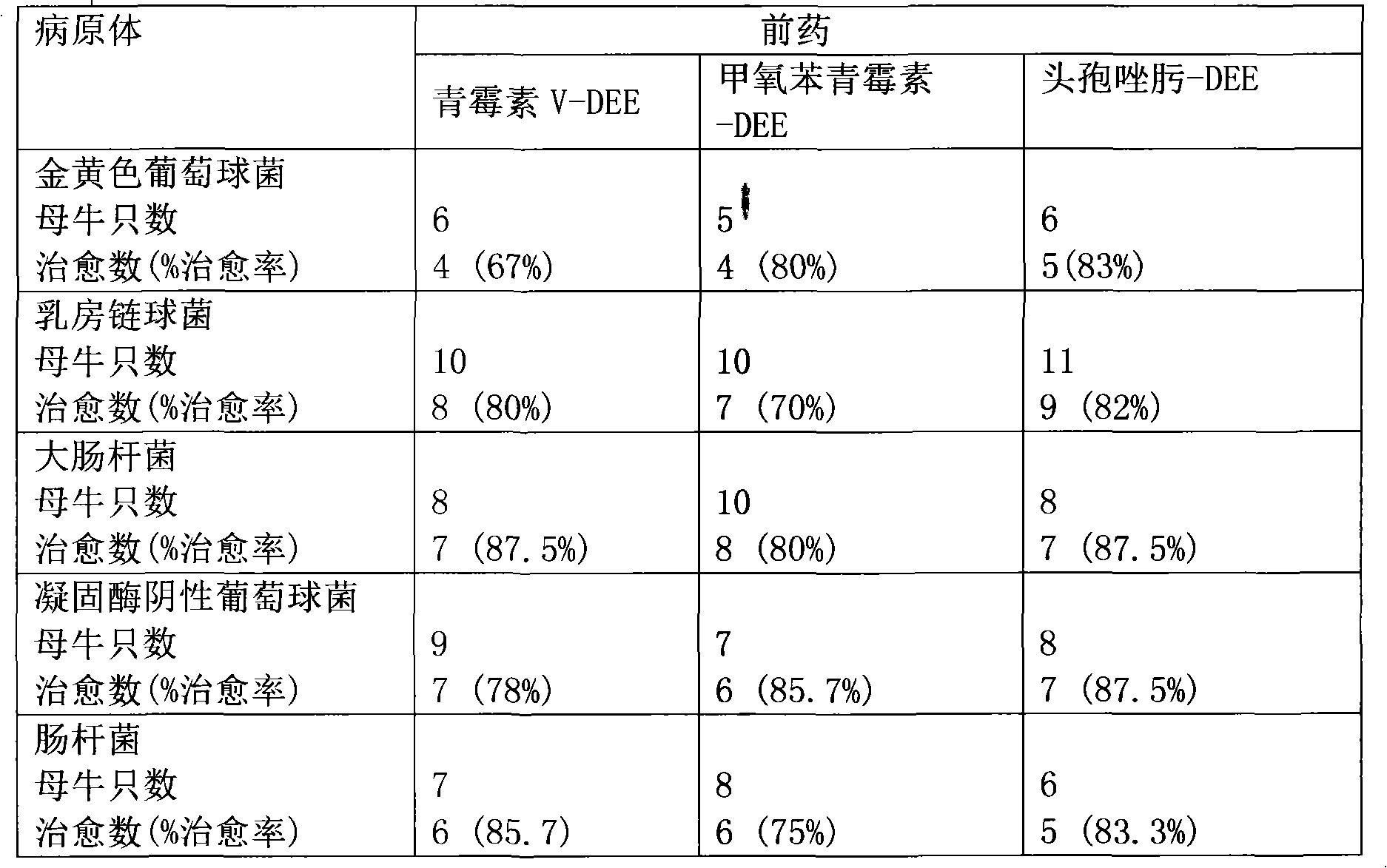
[0043] This example tests the therapeutic effect of the present invention on lactating cows with mastitis.
[0044] Select 90 lactating cows, clinical cure refers to the disappearance of clinical symptoms (one day after the complete disappearance of clinical symptoms is considered clinical cure), in other words, recovery of food intake, rectal temperature <39.0°C, good general condition, and disappearance of udder edema , Milk properties are normal, milk production is normal. Spray 500 mg of 6-paraacetophenoxyaminopenicillinic acid-2-diethylaminoethyl hydrochloride (penicillin V-DEE), 6-( 2,6-dimethoxybenzamide) penicillin acid-2-diethylaminoethyl ester hydrochloride (methicillin-DEE) or 7-[[(2-acetylamino-4-thiazolyl)( Methoxyimino)acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid-2-diethylaminoethyl ester salt salt (Ceftizoxime-DEE).
[0045] Table 1: Clinical cure rate of mastitis in cows with new topical antibiotic prodrugs
[0046]
[0047] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com