Sulfonium salt photo-acid generator using stilbene as main body and preparation method thereof
A technology of stilbene and photoacid generator, which is applied in the fields of optomechanical equipment, photoengraving process of pattern surface, photosensitive material for optomechanical equipment, etc. The two-photon absorption cross-section value is small and other problems, to achieve the effect of good solubility, good acid production performance and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
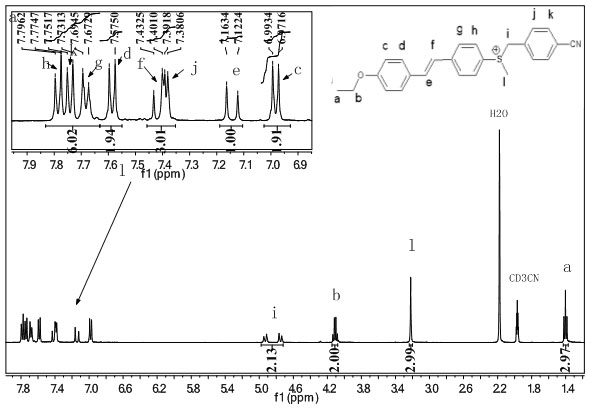
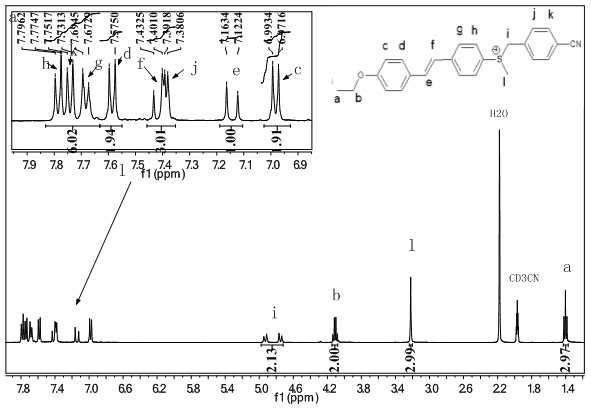
[0030] The initial substance is 4-hydroxybenzaldehyde, the intermediate 4-alkoxystyrene is obtained by Williamson reaction and Wittig reaction, and then it is connected with 4-bromoanisole sulfide by Heck reaction, and then the photogenerated stilbenes are obtained. Acid agent; R in the present embodiment 1 is ethoxy, R 2 is 4-cyanobenzyl, R 3 It is hexafluorophosphate, and its synthetic route is as follows:
[0031]
[0032] In the formula:
[0033] i: ethyl iodide, potassium carbonate, acetone, reflux for 4 hours;
[0034] ii: Methyl triphenylphosphine bromide, sodium hydride, dimethyl sulfoxide, room temperature, reaction for 2 hours;
[0035] iii: 4-bromoanisole sulfide, palladium acetate, triethanolamine, 120°C, react for 24 hours;
[0036] iv: Silver trifluoromethanesulfonate, 4-cyanobenzyl bromide, potassium hexafluorophosphate, dichloromethane, room temperature, react for 24 hours.
[0037] 1. Preparation of 4-ethoxybenzaldehyde
[0038] Dissolve 6.0g of 4-h...
Embodiment 2
[0049] The preparation method is the same as in Example 1, except that when preparing the target photoacid, 4-cyanobenzyl bromide is replaced by an equivalent amount of methyl iodide, and the remaining steps remain unchanged. After the treatment, the target photoacid generator substituted by methyl is synthesized. .
Embodiment 3
[0051] The preparation method is the same as in Example 1, except that ethyl iodide is replaced by methyl iodide when preparing alkoxybenzaldehyde, and the remaining steps are inconvenient. After careful treatment, the target photoacid generator substituted by 4-methoxy is synthesized.
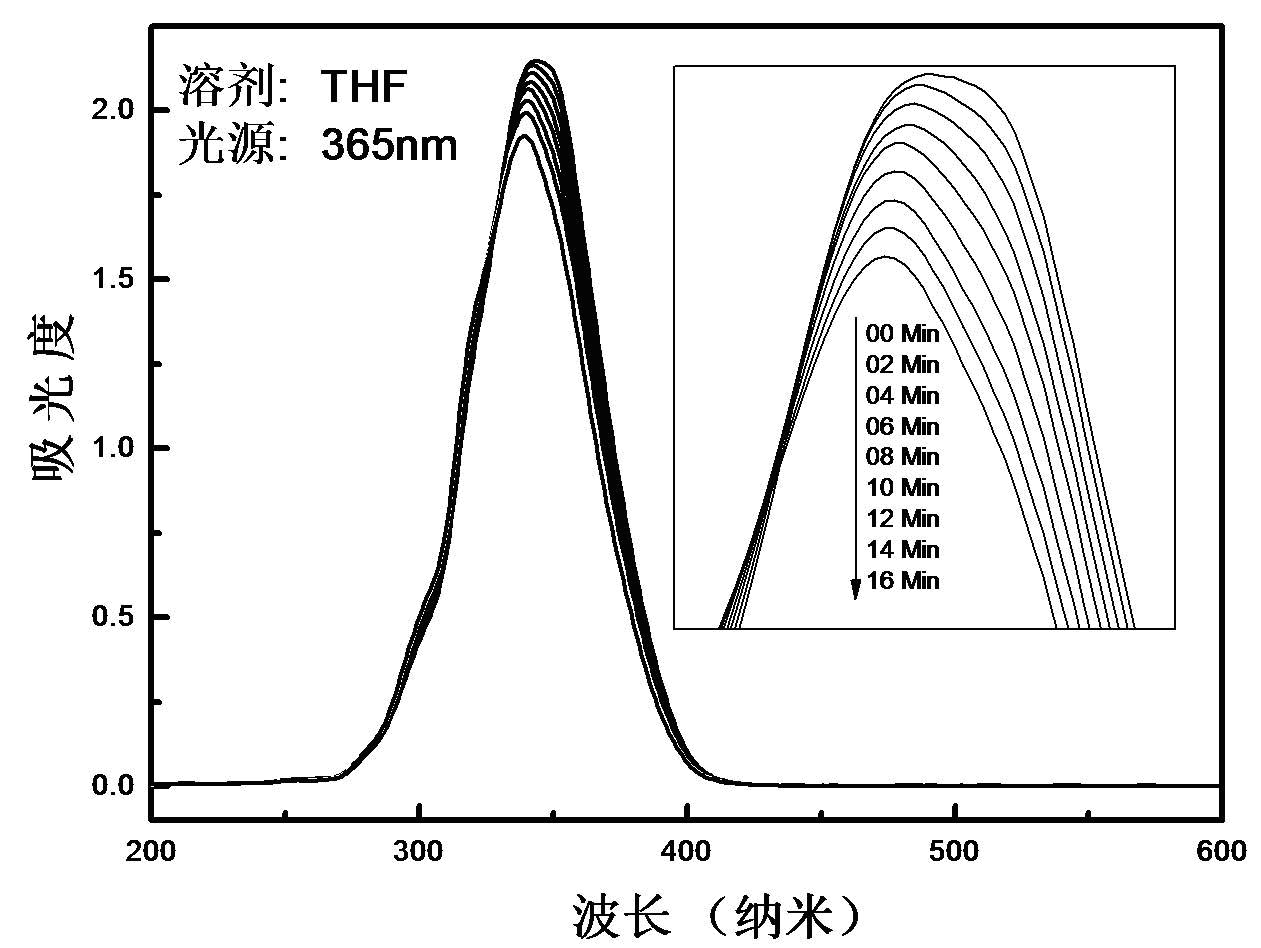
[0052] Using Rhodamine B as the acid indicator and 365nm ultraviolet light as the excitation light source, the photoacid quantum yields of the substances in Example 1-Examples were measured to be 0.40, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com