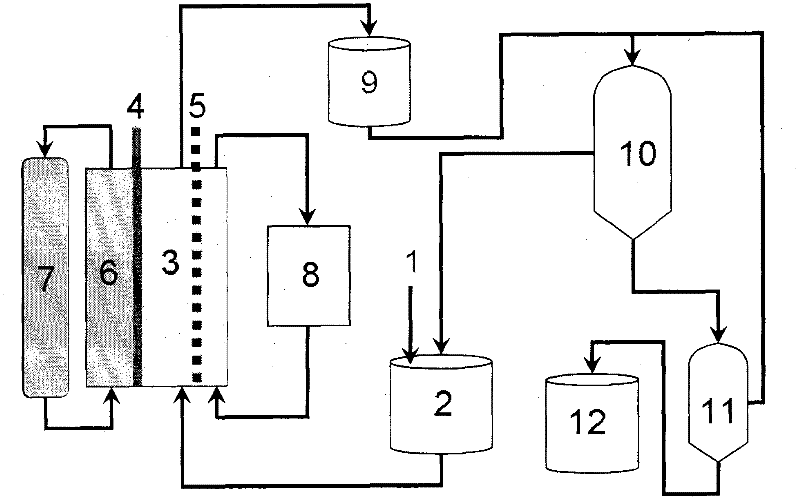
Method and device for producing potassium iodate through oxygen cathode non-diaphragm electrolysis
A technology of potassium iodate and cathode, which is applied in the field of electrochemical electrolysis to prepare potassium iodate, can solve the problems of product pollution, reduced current efficiency and high cell voltage, and achieves the effects of reducing production cost, improving production efficiency and avoiding reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A plate and frame electrolytic cell is used, titanium ruthenium is used for the anode, oxygen cathode is used for the cathode, cobalt oxide is used as the cathode catalyst, and the effective area of the electrodes is 20cm. 2 , prepare KI 100g / L, KOH 80g / L electrolyte 100ml, electrolyte temperature 45°C, cathode gas chamber into oxygen, current density 100mA / cm 2 , electrolysis time 5h, the average cell voltage 0.675V.
[0026] After electrolysis, the electrolytic solution is heated and concentrated, then cooled and crystallized, and the solid obtained by filtering is dried, and the obtained KIO 3 The solid crude product is washed with ice water, recrystallized and refined, and the mother liquor of the crude crystal and recrystallization is combined, and the KIO in the mother liquor is obtained by titration analysis. 3 Mass 2.15g, solid KIO 3 The mass is 10.38g, and the analyzed purity is 99.78%. All KIOs 3 Yield 97.2%, solid KIO 3 Yield 80.52%, unit KIO 3 The po...
Embodiment 2
[0028] Use a plate and frame electrolytic cell, titanium ruthenium is used for the anode, oxygen cathode is used for the cathode, and the cathode catalyst is Co 3 o 4 , the electrode effective area is 20cm 2 , prepare KI 180g / L, KOH 100g / L electrolyte 200ml, electrolyte temperature 75°C, cathode gas chamber into oxygen, current density 200mA / cm 2 , electrolysis time 9h, the average cell voltage 0.714V.
[0029] After electrolysis, the electrolytic solution is heated and concentrated, then cooled and crystallized, and the solid obtained by filtering is dried, and the obtained KIO 3The solid crude product is washed with ice water, recrystallized and refined, and the mother liquor of the crude crystal and recrystallization is combined, and the KIO in the mother liquor is obtained by titration analysis. 3 Mass 7.08g, solid KIO 3 The mass is 38.14 g, and the analyzed purity is 99.46%. All KIOs 3 Yield 97.44%, solid KIO 3 Yield 82.18%. UnitKIO 3 The power consumption of ele...
Embodiment 3
[0031] Use a plate and frame electrolytic cell, nickel ruthenium is used for the anode, oxygen cathode is used for the cathode, the cathode catalyst is Ag, and the effective area of the electrodes is 20cm 2 , prepare KI 160g / L, KOH 100g / L electrolyte 200ml, electrolyte temperature 75°C, cathode gas chamber into oxygen, current density 200mA / cm 2 , electrolysis time 8h, the average cell voltage 0.764V.
[0032] After electrolysis, the electrolytic solution is heated and concentrated, then cooled and crystallized, and the solid obtained by filtering is dried, and the obtained KIO 3 The solid crude product is washed with ice water, recrystallized and refined, and the mother liquor of the crude crystal and recrystallization is combined, and the KIO in the mother liquor is obtained by titration analysis. 3 Mass 6.71g, solid KIO 3 The mass is 33.62 g, and the analyzed purity is 99.92%. All KIOs 3 Yield 97.76%, solid KIO 3 Yield 81.5%, unit KIO 3 The power consumption of elec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com