Mutant venin fibrinolytic enzyme gene and preparation method thereof
A technology of fibrinolytic enzyme and gene, applied in the field of mutant snake venom fibrinolytic enzyme gene and its preparation, can solve the problems of re-embolization, oxidation, and limited sources of raw materials, and achieve the effect of eliminating cumbersome steps and being easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Utilizing the amino acid sequence of the fibrolase protein (GenBank No.P28891) published by the American National Center for Biotechnology (NCBI) database, the QQR at its N-terminus was mutated into serine S, and its amino acid sequence is shown in SEQ ID No. .1 shown. Combined with the codon bias of eukaryotic organisms, the nucleotide sequence is designed, and the nucleotide sequence is shown in SEQ ID No.2.
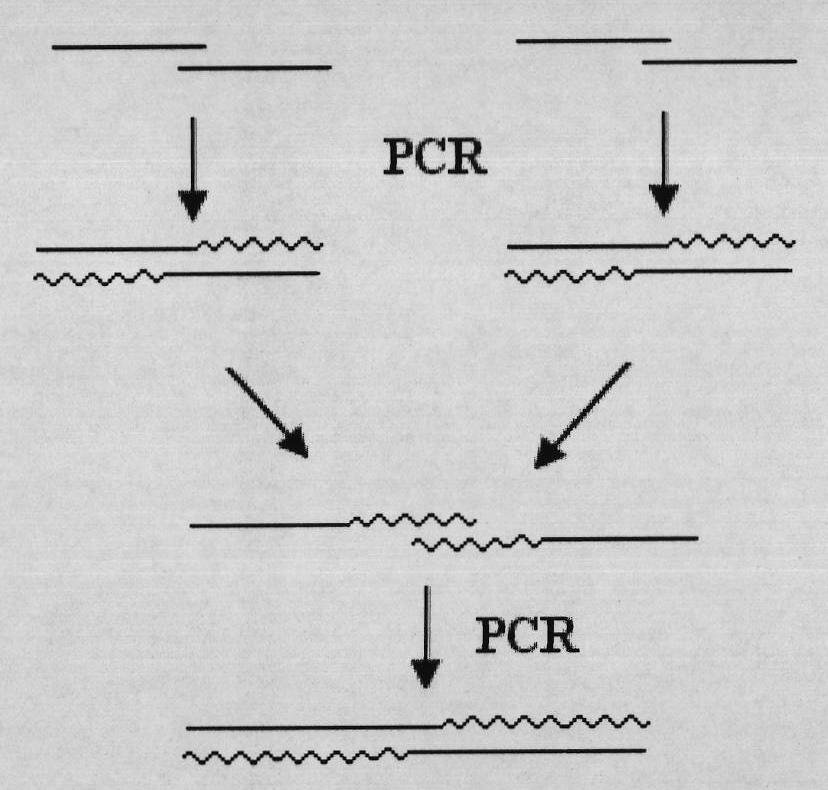
[0025] Four rounds of PCR reactions were used to obtain the gene fragment encoding Alfimeprase containing the required restriction enzyme site, His×6 tag, and EK site. The PCR schematic diagram is as follows figure 1 shown. The reaction system used in each round of PCR is as follows:
[0026] The first round of PCR with SEQ ID No.3 and SEQ ID No.4, SEQ ID No.5 and SEQ ID No.6, SEQ ID No.7 and SEQ ID No.8, SEQ ID No.9 and SEQ ID No.10, SEQ ID No.11 and SEQ ID No.12, SEQ ID No.13 and SEQ ID No.14, SEQ ID No.15 and SEQ ID No.16 are primers for PCR amplification ...
Embodiment 2
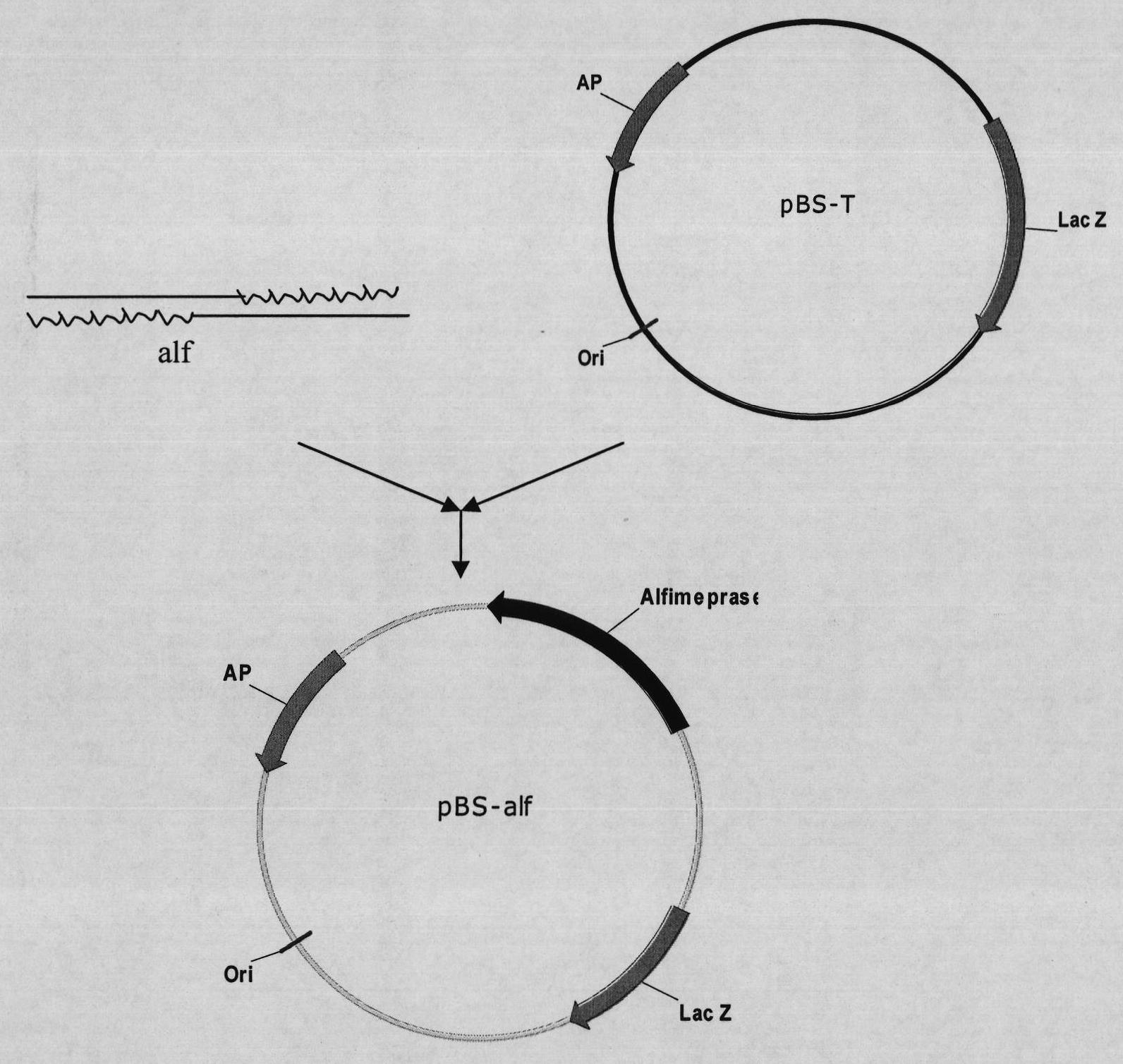
[0086] according to Figure 4 The method shown was used to construct the expression vector pPIC9K-His-Alf. The pBS-Alf constructed in Example 1 and the expression vector pPIC9K were digested with EcoR I and Not I ( Figure 5 ), purify and collect the digested fragments, and connect them to obtain the recombinant expression vector pPIC9K-His6-Alf. The double digestion and connection system are as follows:
[0087] Enzyme digestion reaction system 1: Enzyme digestion reaction system 2:
[0088] pBS-Alf plasmid 10μl pPic9k plasmid 10μl
[0089] EcoR I (12u / μl) 2.0μl EcoR I (12u / μl) 1.5μl
[0090] Not I (10u / μl) 2.0μl Not I (10u / μl) 1.5μl
[0091] 10x Digestion Buffer 6μl 10x Digestion Buffer 6μl
[0092] 10xBSA 6μl 10xBSA 6μl
[0093] Sterile water 34μl Sterile water 34.5μl
[0094] Total volume 60μl Total volume 60μl
[0095] Connection reaction system:
[0096] Recover 1.5 μl of purified pPIC9K plasmid
[0097] Recover and purify the target fragment His6-Alf 5 μl
[...
Embodiment 3
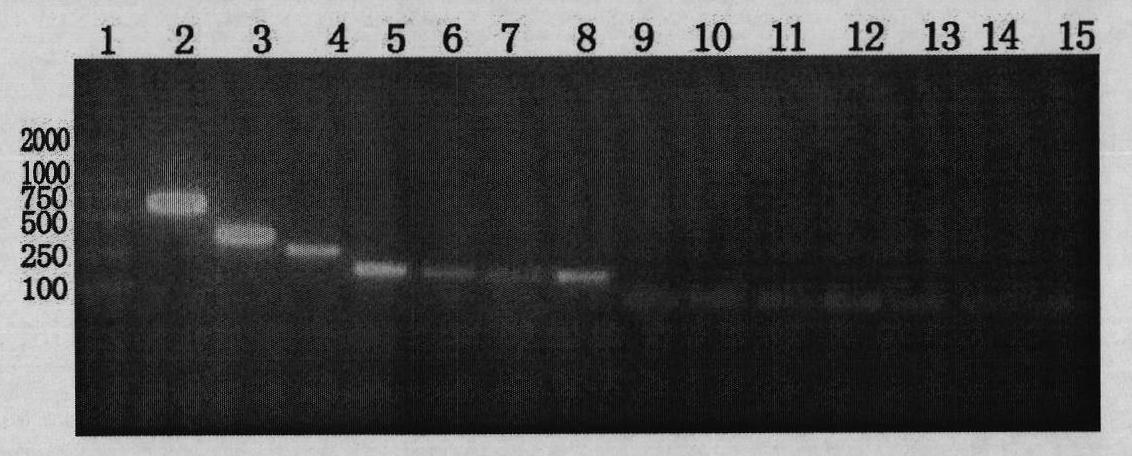
[0103] Shake flask culture in BMG / MY liquid medium (recipe: 1% yeast extract, 2% peptone, 100mM potassium phosphate pH6.0, 0.00004% biotin, 1% glycerol, 0.5% methanol.), liquid volume 100ml, shaker speed 250rpm, when the OD600 value reaches 1-1.5, add sterile methanol 1-2ml to induce expression, and then add once every 24 hours. Sampling was carried out continuously for electrophoresis determination, and cultured for 5 days.
[0104] Collect the fermentation broth, centrifuge at 10,000 rpm, concentrate in vacuum at low temperature, gradually add ammonium sulfate to the concentrated solution to a final concentration of 50%, and keep stirring gently, centrifuge the precipitate at 3,000 rpm, remove the supernatant, and redissolve the precipitate with physiological saline. The solution was purified according to the operating instructions of the His-Bind protein purification kit from Novagen. The purified protein solution was collected, cut with enterokinase EK, precipitated with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com