Method for absorbing and fixing carbon dioxide through mineral carbonization
A technology of carbon dioxide and minerals, applied in the direction of silicon dioxide, chemical instruments and methods, silicon oxide, etc., can solve the problems that cristobalite cannot be separated out, affect the physical and chemical properties of montmorillonite, etc., and achieve high value-added development and utilization, good ecology Benefits, simple and feasible process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
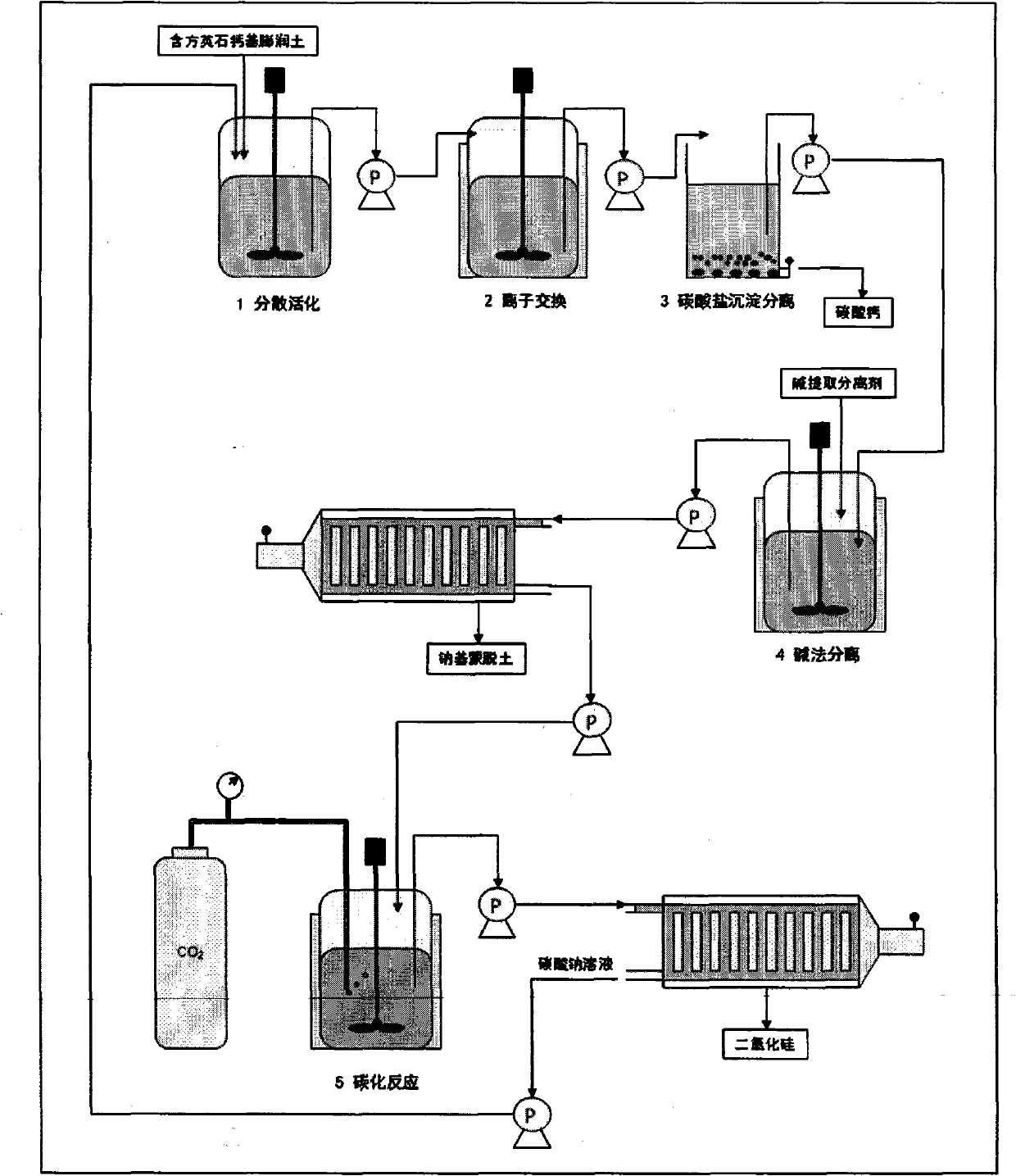
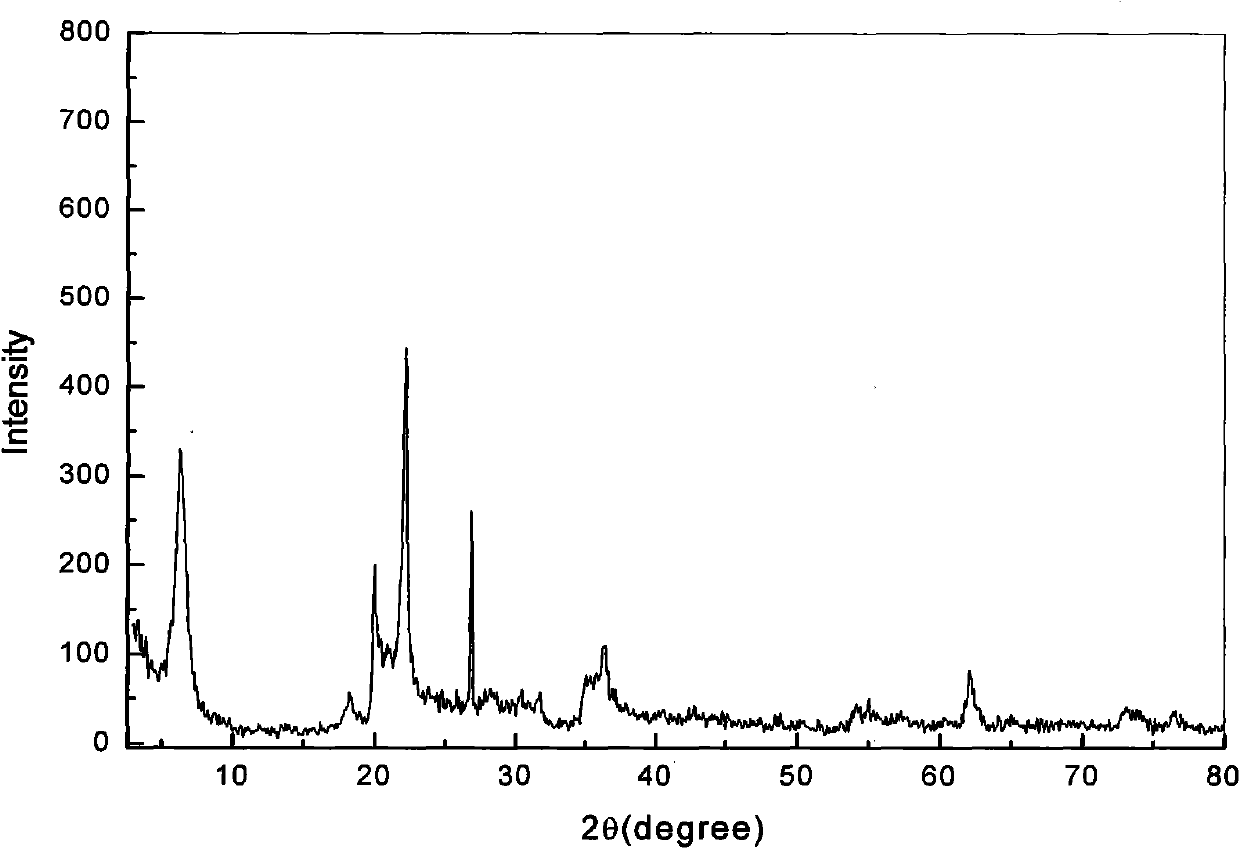
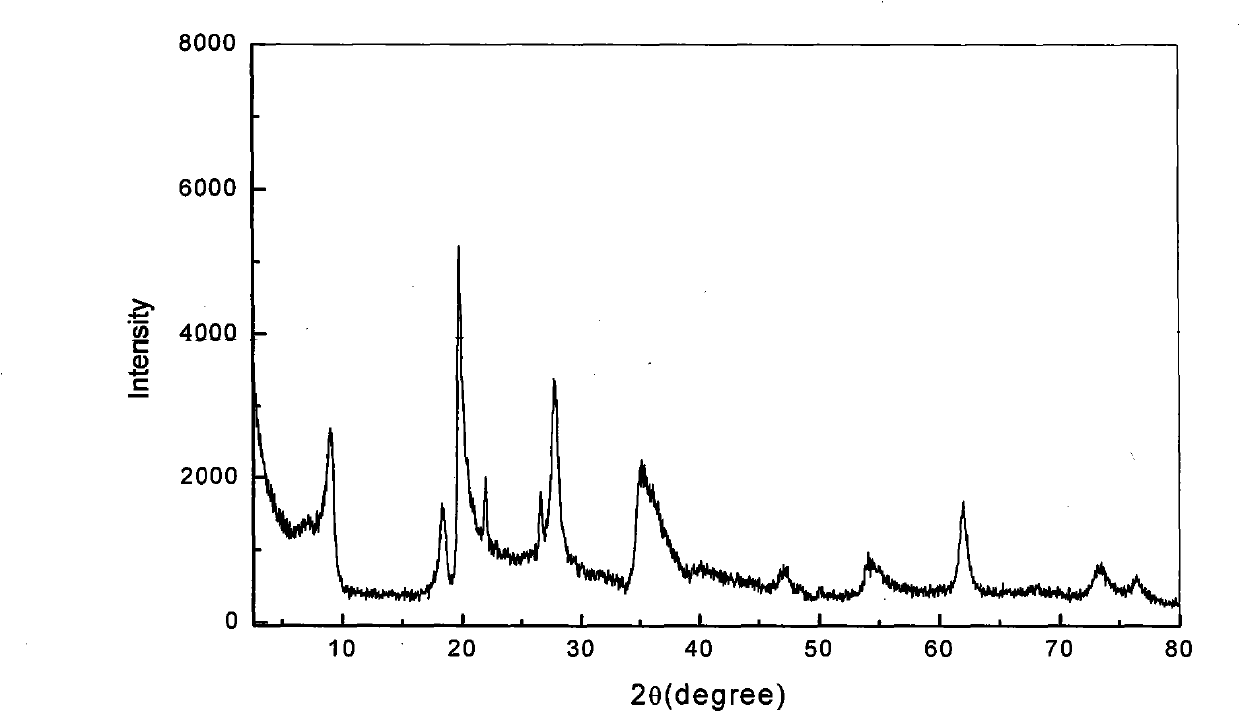
[0029] The ore composition of calcium-based bentonite is 61.4% of montmorillonite; 28.9% of cristobalite; 5.3% of quartz; 4.4% of feldspar (see X-ray diffraction pattern figure 2 , The position of the diffraction peak on the 001 plane of bentonite (2θ=6.36°) shows that the exchangeable cation between the bentonite layers is calcium ion). Add aqueous sodium carbonate solution (press filtrate after carbonization reaction) to the coarsely crushed natural calcium-based bentonite containing cristobalite impurities, fully disperse and carry out ion exchange reaction, the temperature of ion exchange reaction is 90 ° C, and the reaction time is 2 hours;
[0030] Centrifugal separation removes calcium carbonate particles and other large particles of impurities formed after the ion exchange reaction. Add 0.5 mol / L sodium hydroxide aqueous solution to the obtained bentonite slurry for alkali extraction and separation. The amount added is 1 / 5 of the mass of bentonite. The reaction temper...
Embodiment 2
[0033] The raw ore composition of calcium-based bentonite and other steps are the same as in Example 1.
[0034] In the carbon dioxide carbonation fixation reaction, the ventilation rate was 10L / min, the ventilation pressure was 0.35MPa, the carbonization reaction temperature was controlled at 60°C, and the reaction time was 45 minutes. After the carbonization fixation reaction, the carbon dioxide fixation rate was determined to be 63% by measuring the amount of carbonate in the system.
Embodiment 3
[0036] The raw ore composition of calcium-based bentonite and other steps are the same as in Example 1.
[0037] In the carbon dioxide carbonation fixation reaction, the ventilation rate was 5L / min, the ventilation pressure was 1.0MPa, the carbonization reaction temperature was controlled at 50°C, and the reaction time was 15 minutes. After the carbonization fixation reaction, the carbon dioxide fixation rate was determined to be 68% by measuring the amount of carbonate in the system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com