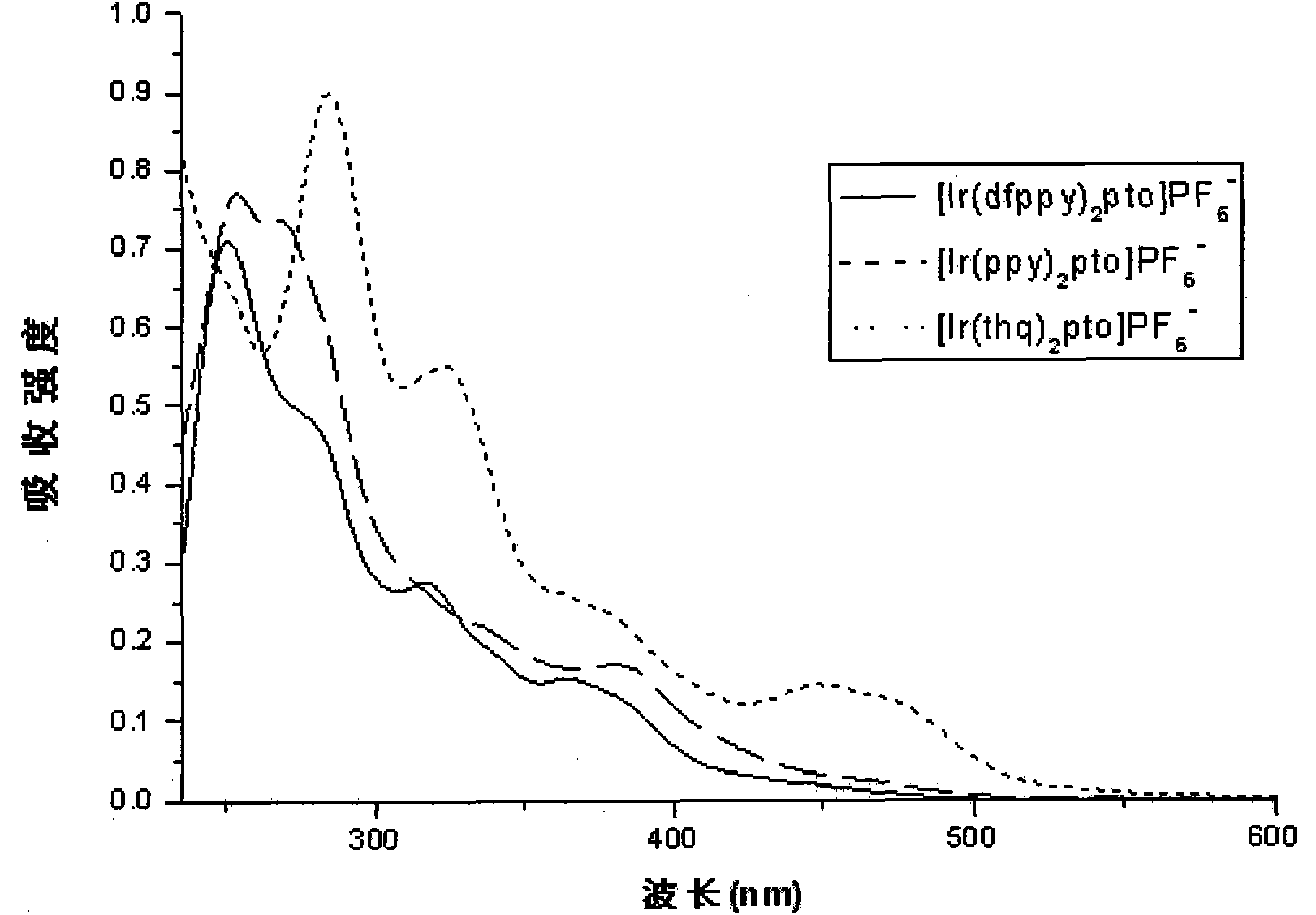
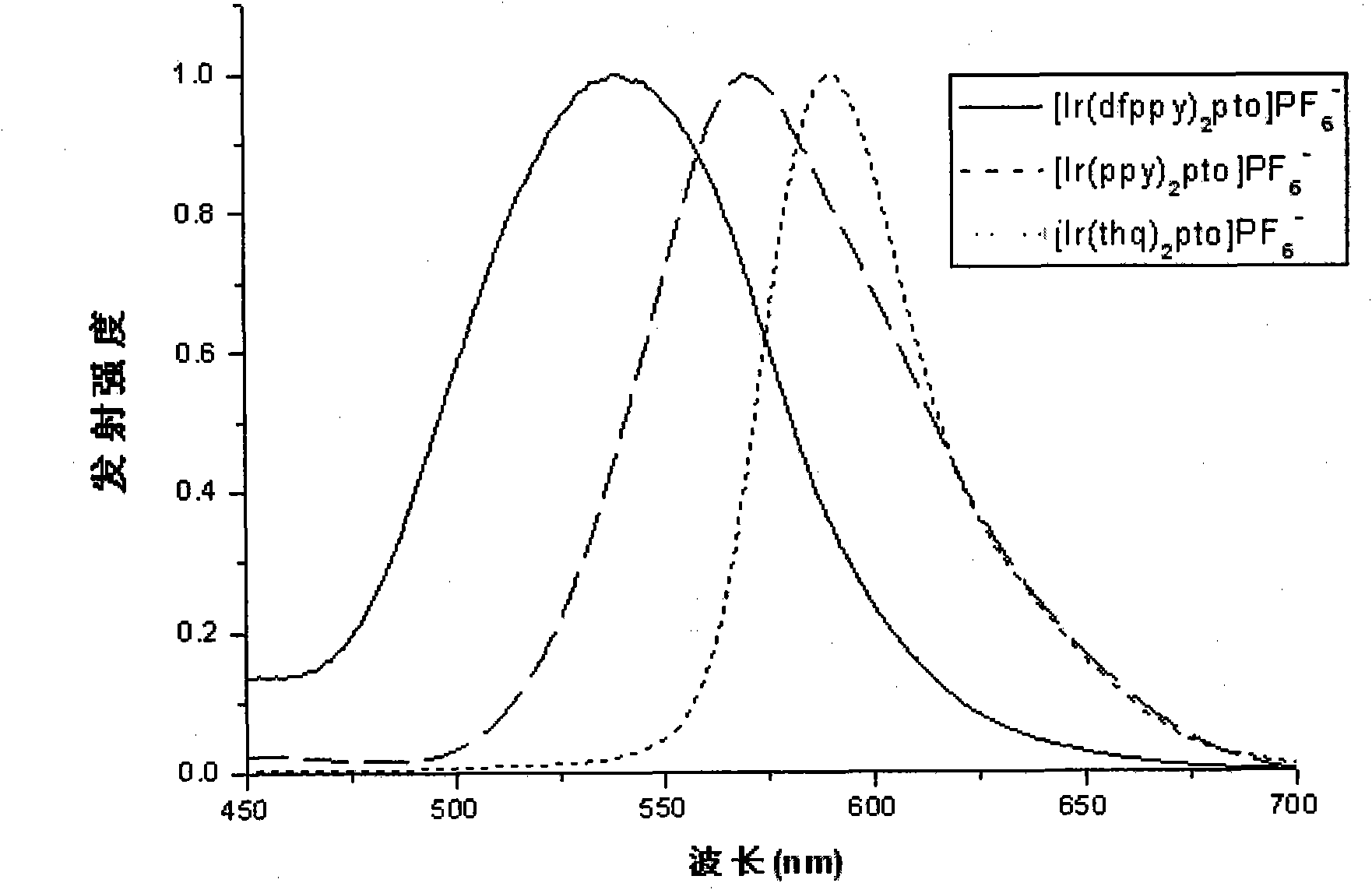
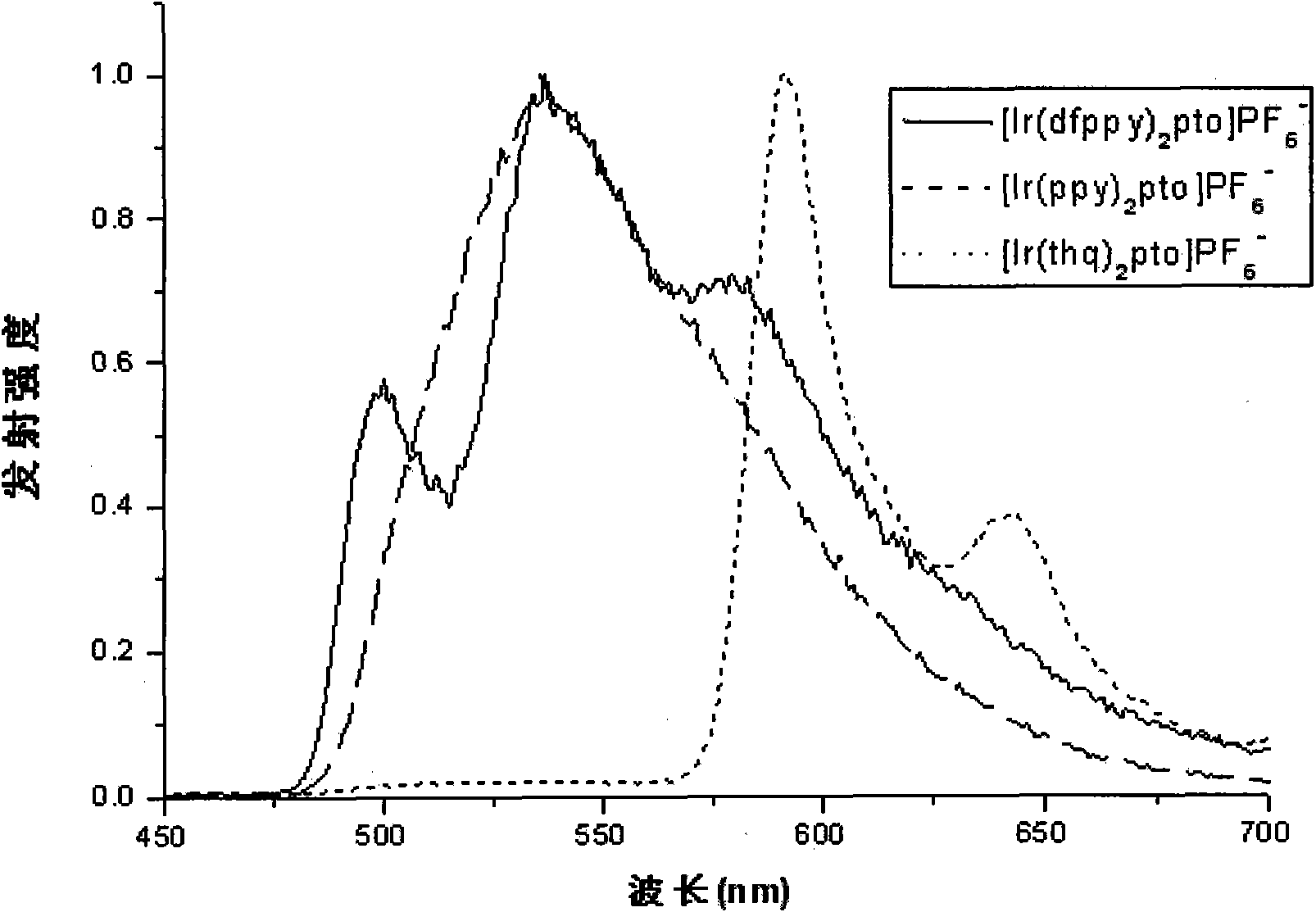
Preparation and applications of water-soluble phosphorescnet iridium complex
A technology of iridium complex and phosphorescence emission, which is used in compounds containing elements of Group 8/9/10/18 of the periodic table, organic chemistry, biological testing, etc. problem, to achieve the effect of improving emission wavelength tunability, reducing cytotoxicity, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, the preparation of functional N^N ligand:
[0023] Compound 1: Preparation of 5-nitro-1,10-phenanthroline
[0024] Add 5g of 1,10-phenanthroline hydrate into a round bottom flask, add 30mL of concentrated sulfuric acid to dissolve, then add 15mL of fuming nitric acid dropwise, heat the solution to keep its temperature at 160-170°C, and reflux for 2 hours. After the reaction was complete, it was allowed to cool to room temperature, after which the solution was poured into ice water. The pH was adjusted to 3 by adding 10% sodium hydroxide solution. The precipitated yellow precipitate was the product, which was filtered off with suction, washed with water and dried in vacuo. Yield 5 g (88%) of a pale yellow solid. GC-MS (EI-m / z): 225 (M + )
[0025] Compound 2: Preparation of 5-amino-1,10-phenanthroline
[0026] Dissolve 1.889g of 5-nitro-1,10-phenanthroline in 150mL of absolute ethanol, add 0.378g of 5% Pd / C catalyst, heat to 70°C under nitrogen protec...
Embodiment 2
[0030] Embodiment 2: the preparation of iridium complex containing hydrophilic group:
[0031] Compound 1: Iridium complex [Ir(dfppy) 2 cpa] + PF 6 - preparation of
[0032] Weigh iridium dichloro bridge compound 121mg and N^N ligand (cpa) 68mg and join in the double-necked bottle, then add 15mL CH 2 Cl 2 Mixed solvent with methanol (2:1, v / v), reflux under magnetic stirring. After 4 hours, it was cooled to room temperature, and 5 times the equivalent of potassium hexafluorophosphate (KPF 6 ), after continuing to stir for about 1 hour, the solvent was removed by rotary evaporation under reduced pressure, and the resulting solid mixture was redissolved in about 10 mL of CH 2 Cl 2 , the insoluble matter was removed by filtration, and the filtrate was rotary evaporated under reduced pressure to remove the solvent, and the solid obtained was separated by column chromatography (dichloromethane / acetone) to obtain a pure product. Yield: 63%, 1 HNMR (400MHz, CDCl 3 )9.32(s,...
Embodiment 3
[0037] Example 3: Live cell imaging experiment:
[0038] The iridium complex was prepared into a 10 mmol solution, and 20 uL of the solution was pipetted into 2000 uL of PBS buffer solution to dilute the concentration to 10 μM. Take 2 mL of the solution and incubate the cells for 30 minutes, then wash the cells with PBS buffer for 3-5 times, and use a wavelength of 405 nm to excite the cells for confocal imaging and Z-scan. The test data shows that the water-soluble iridium complex has good cell penetration and is distributed in the cytoplasmic area.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com