Ratiometric fluorescent probe and application thereof
A fluorescent probe, ratiometric technology, applied in the direction of fluorescence/phosphorescence, luminescent materials, chemical instruments and methods, etc., can solve the problems of being easily photobleached, the accuracy of the results, and the poor specificity of the probe, so as to improve the specificity High stability and sensitivity, high stability, and the effect of eliminating interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Ligand [4'-(2,4-dimethoxyphenyl)-2,2':6'2"-biterpyridine-6,6"-dimethylamine]tetraacetic acid ( Synthesis of DTTA for short) and its methyl acetate (AM-DTTA for short).
[0031] 1. Synthesis of DTTA
[0032] Synthetic route such as figure 1 As shown, the basic operation process is as follows:
[0033] (1) Synthesis of 4'-(2,4-dimethoxyphenyl)-2,2':6',2"-biterpyridine (compound 1)
[0034] 3.32 grams of 2,4-dimethoxybenzaldehyde (20mmol), 4.84 grams of 2-acetylpyridine (40mmol), and 2.64 grams of KOH (85%, 40mmol) were mixed and dissolved in 100mL of absolute ethanol, and then 50mL of concentrated ammonia was added , stirred at 50°C for 10 hours, and the precipitate was collected by filtration. The crude product was recrystallized from ethanol to obtain 3.61 g of the target compound, with a yield of 48.9%. 1 H NMR (CDCl 3 ) measurement results: δ=8.71 (d, J, 4.8Hz, 2H); 8.66 (d, J, 7.6Hz, 2H); 8.62 (s, 2H); 7.86 (t, J, 7.6Hz, 2H); 7.49 (d, J, 8.4Hz, 1H...
Embodiment 2
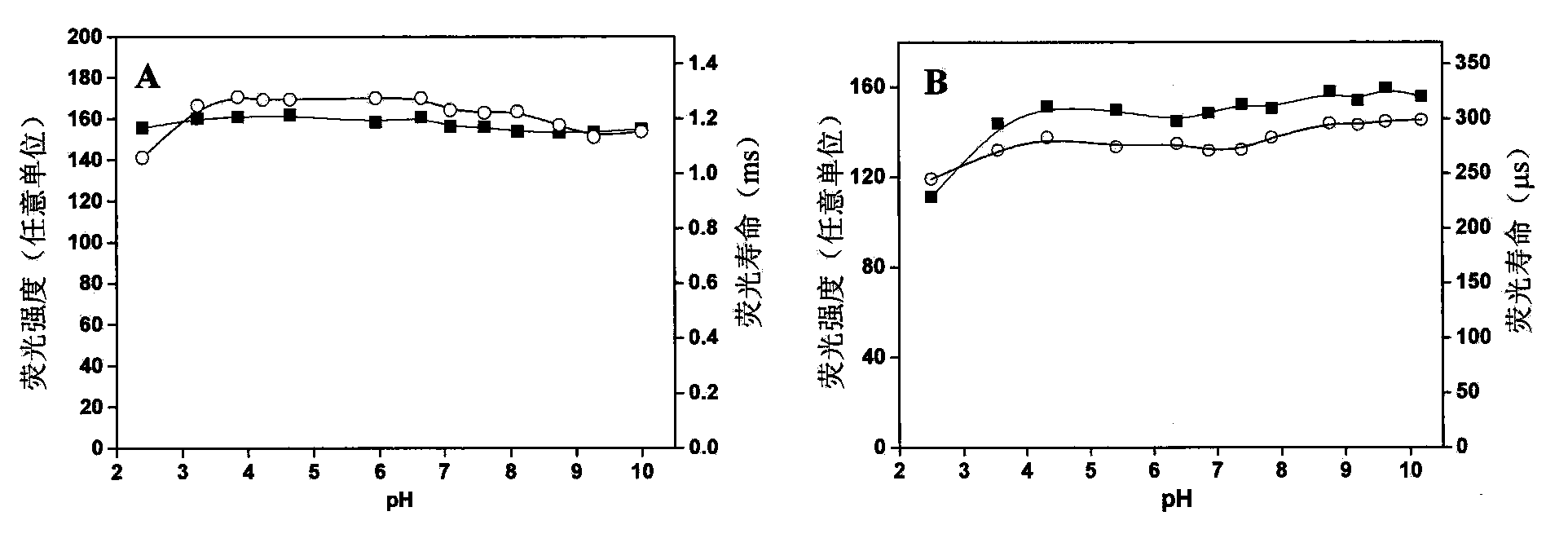
[0050] Example 2: Two rare earth complexes DTTA-Eu 3+ and DTTA-Tb 3+ Determination of properties
[0051] 1. Spectral properties
[0052] DTTA-Eu was determined using 0.05mol / L boric acid buffer solution with pH value 9.1 as solvent 3+ and DTTA-Tb 3+ (The molar ratio of ligand and metal ion in the two complexes is 1:1) UV-Vis absorption spectrum, fluorescence spectrum, molar extinction coefficient (ε), fluorescence quantum yield (φ) and fluorescence lifetime (τ). The instrument used for the determination of ultraviolet-visible absorption spectrum is a Perkin Elmer Lambda 35 spectrophotometer. The fluorescence measurement instrument is a Perkin Elmer LS 50B fluorescence spectrophotometer. Fluorescence quantum yield measurement using 4’-phenyl-2,2’:6’,2”-tripyr-6,6”-dimethylaminetetraacetic acid and Eu 3+ and Tb 3+ The complex is measured as a standard according to the literature method (document 14: M.Latva, H.Takalo, J.Kankare, J.Lumin.1997, 75, 149), and the calculatio...
Embodiment 3
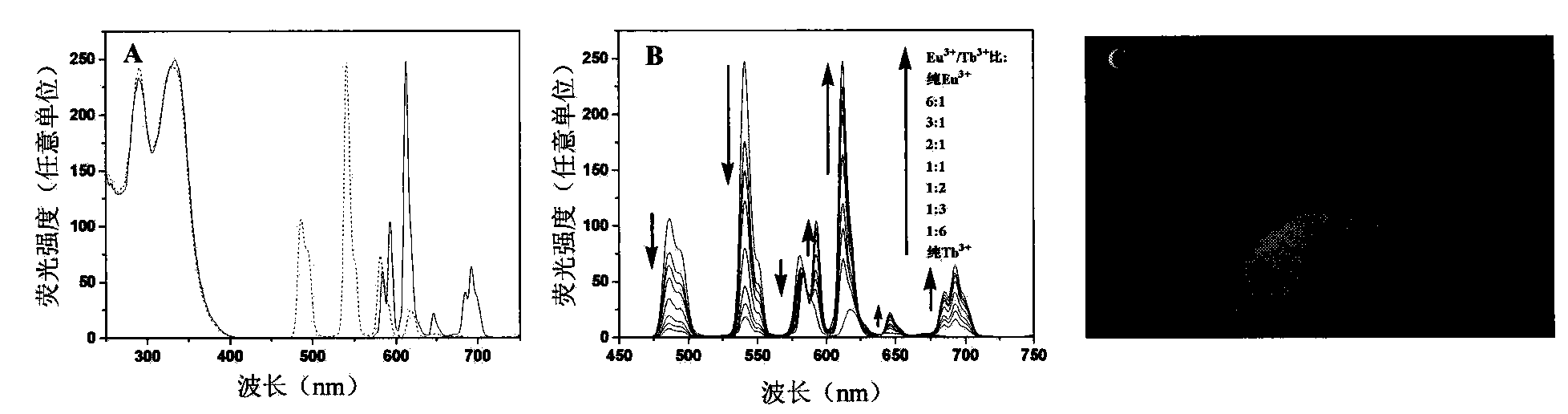
[0058] Example 3: with DTTA-Eu 3+ / Tb 3+ As a fluorescent probe for the determination of ONOO in aqueous solution - concentration
[0059] 1. ONOO - to DTTA-Eu 3+ with DTTA-Tb 3+ Effect of Fluorescent Properties
[0060] Add DTTA-Ln to a series of 0.05mol / L Tris-HCl buffer solutions with a pH value of 7.4 3+ (Ln 3+ =Eu 3+ or Tb 3+ , 2.0μmol / L) and different concentrations of ONOO - , After stirring and reacting for 15 minutes, the fluorescence intensity and fluorescence lifetime of each solution were measured. The measuring instrument is a PerkinElmer LS 50B fluorescence spectrophotometer.
[0061] Such as Figure 4 As shown, with ONOO - Concentration increases, DTTA-Eu 3+ The fluorescence intensity and fluorescence lifetime basically do not change; but DTTA-Tb 3+ The fluorescence intensity gradually decreases, the fluorescence lifetime is significantly shortened, and the time-resolved fluorescence intensity is significantly weakened. Figure 5 DTTA-Eu is given...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com