Bioactivity detection method and application of recombined humanized neuroglobin
A technology for cerebroglobin and a detection method, applied in the field of biomedicine, can solve the problems of insufficient sensitivity of detection technology, unproven, unsuitable for detection of rhNgb biological activity, etc., and achieves high repeatability of detection data, reliable measurement results, and detection method. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
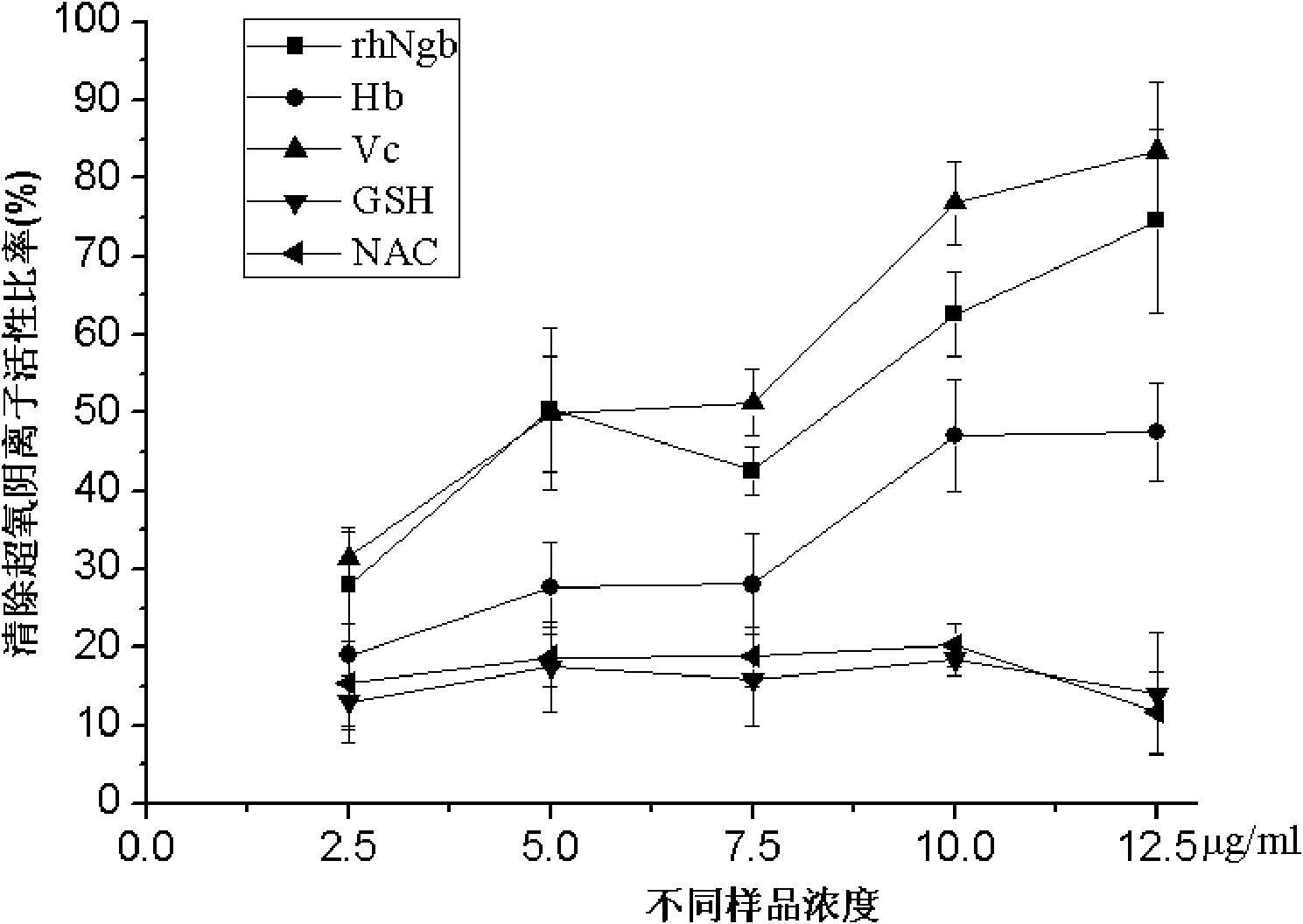
[0041] Example 1: detection of rhNgb scavenging superoxide anion activity
[0042] Nitro blue tetrazolium chloride (NBT)-photoreduction method was adopted to measure, VB in this reaction 2 It reacts with EDTA under light to produce superoxide anion, which reacts with NBT and has a maximum absorption peak at 560nm. In the reaction, VB2 and EDTA react under light to produce superoxide anion, which reacts with NBT. After adding rhNgb, rhNgb reacts with superoxide anion, which further prevents the reaction between superoxide anion and NBT. The value of the absorption peak at will change. According to the reaction degree of rhNgb and superoxide anion, the ability of rhNgb to scavenge superoxide anion can be calculated.
[0043] In the embodiment, the assay method for removing superoxide anion activity is divided into reagent preparation, sample / reference substance preparation, measuring sample and reference substance absorbance, and calculating the activity four steps of removing...
Embodiment 2
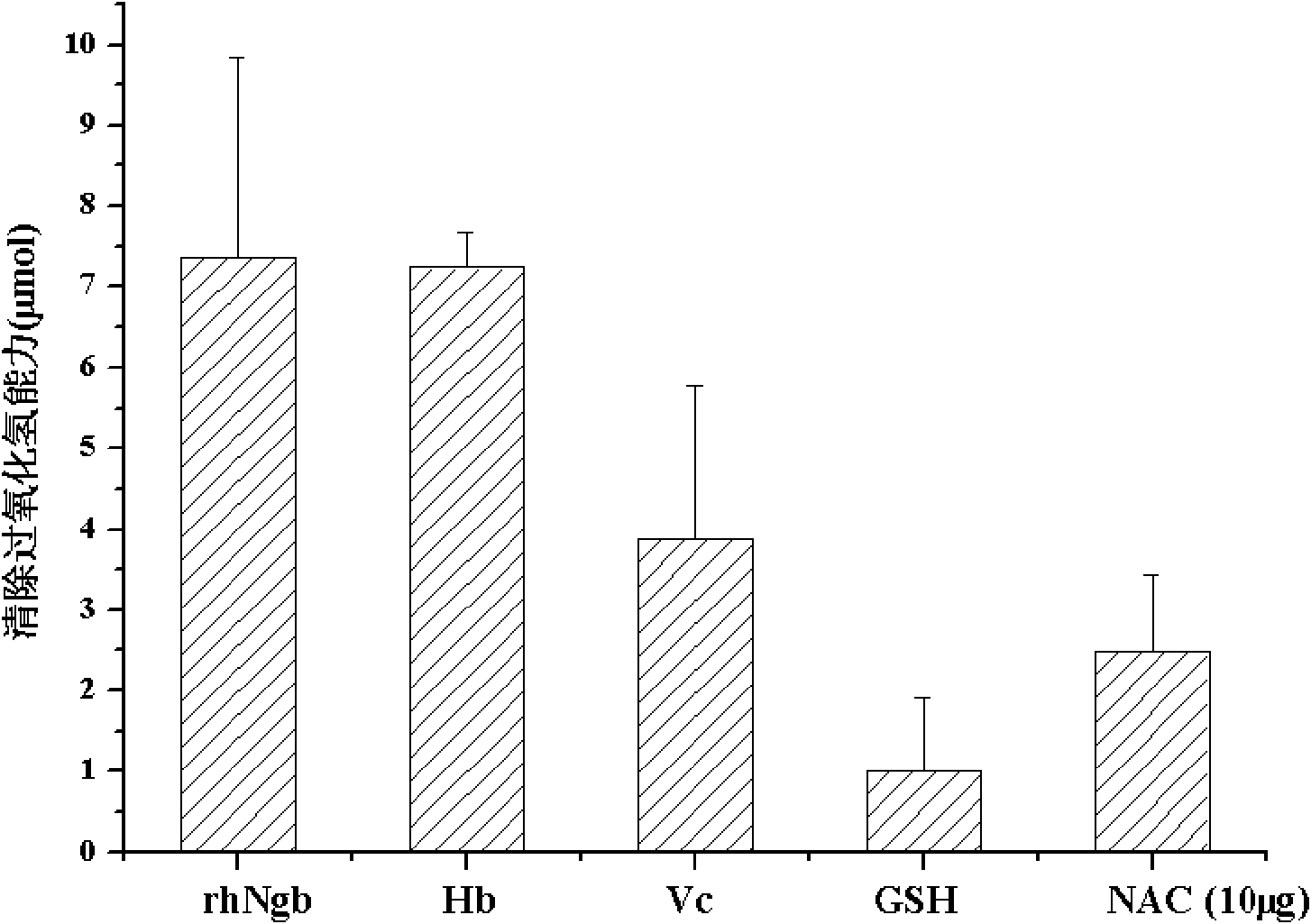
[0059] Example 2: detection of rhNgb scavenging hydrogen peroxide activity
[0060] In the case of relatively sufficient hydrogen peroxide, catalase can catalyze hydrogen peroxide to produce water and oxygen. Residual hydrogen peroxide can produce red product N-(4-antipyrinyl)-3-chloro-5-sulfonic acid group-p-benzoquinone imine (N-(4- antipyryl)-3-chloro-5-sulfonate-p-benzoquinonemonoimine), its maximum absorption wavelength is 520nm.
[0061] The hydrogen peroxide removal activity of rhNgb was detected using the catalase detection kit of Beyond Biotechnology Research Institute.
[0062] 1. Reagent preparation
[0063] (1) Preparation of rhNgb sample solution: On the basis of the prepared rhNgb sample, rhNgb was prepared into a 1 mg / ml sample solution with double distilled water.
[0064] (2) Preparation of the reference substance vitamin C (Vc), reduced glutathione (GSH), N-acetylcysteine (NAC) and hemoglobin (Hb) solution: prepare with rhNgb, weigh 10 mg of the referenc...
Embodiment 3
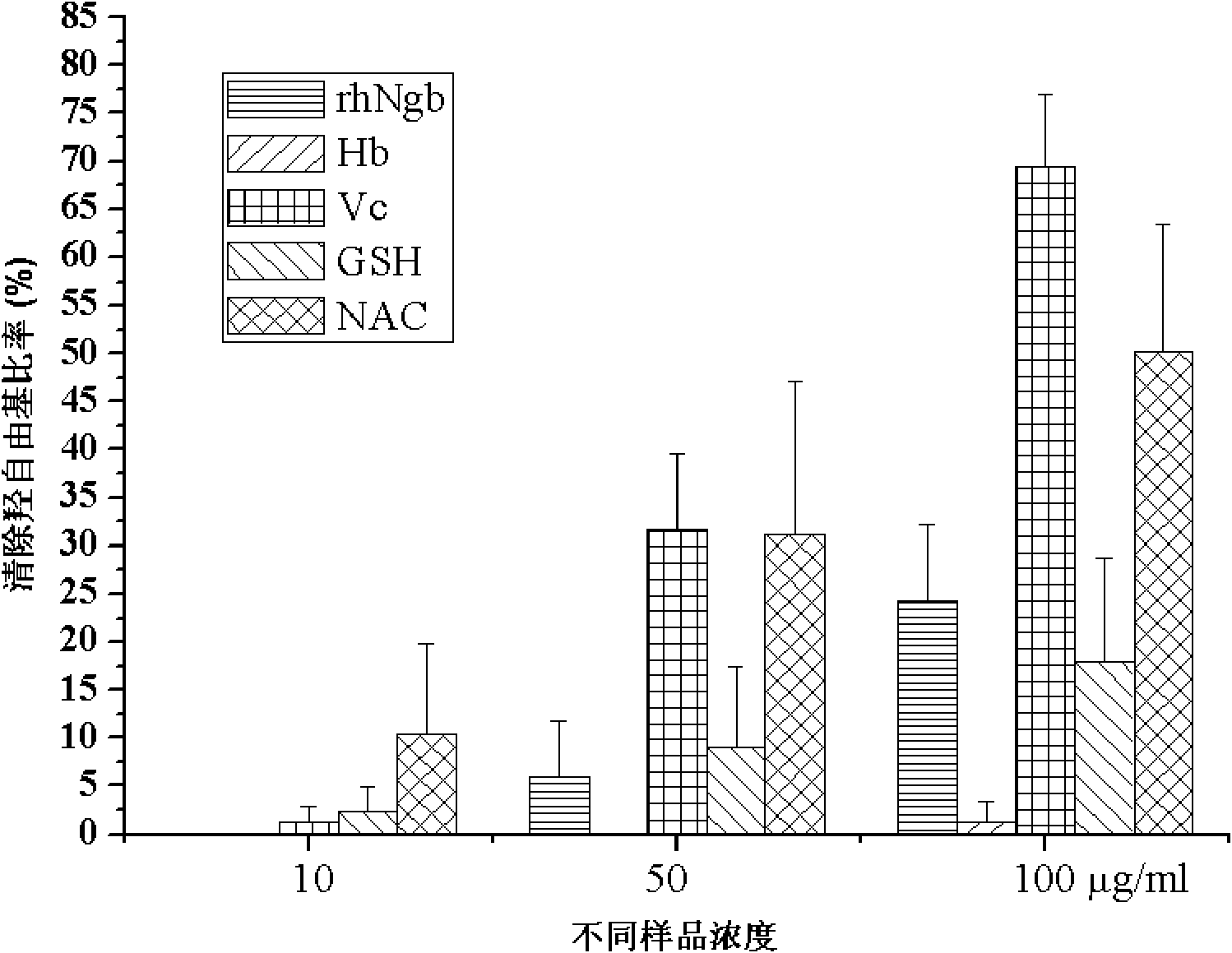
[0083] Example 3: Detection of rhNgb scavenging hydroxyl radical activity
[0084] o-phenanthroline-Fe 2+ The activity of rhNgb to scavenge hydroxyl radicals was determined by colorimetry. Hydroxyl radicals generate Fe through the Fenton reaction 2+ and H 2 o 2 .
[0085] 1) Use double distilled water to prepare rhNgb into sample solutions with different concentrations of 0.1, 0.5, and 1 mg / ml, while hemoglobin (Hb), Vc, reduced glutathione (GSH), N-acetylcysteine (NAC ) solution was prepared in the same way as rhNgb, and stored in a 4°C refrigerator for later use;
[0086] 2) Add 100 μl of 0.75 mM o-phenanthroline solution, 200 μl of PBS (pH7.2), and 0.75 mM FeSO to the microtube (centrifugal Eppendorf tube) 4 100μl and 50μl double distilled water, shake well;
[0087] 3) Add 0.01% H 2 o 2 50μl, start the reaction, and place in a water bath at 37°C for 60min;
[0088] 4) After taking the mixed solution out of the water bath, measure its absorbance at 536nm, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com