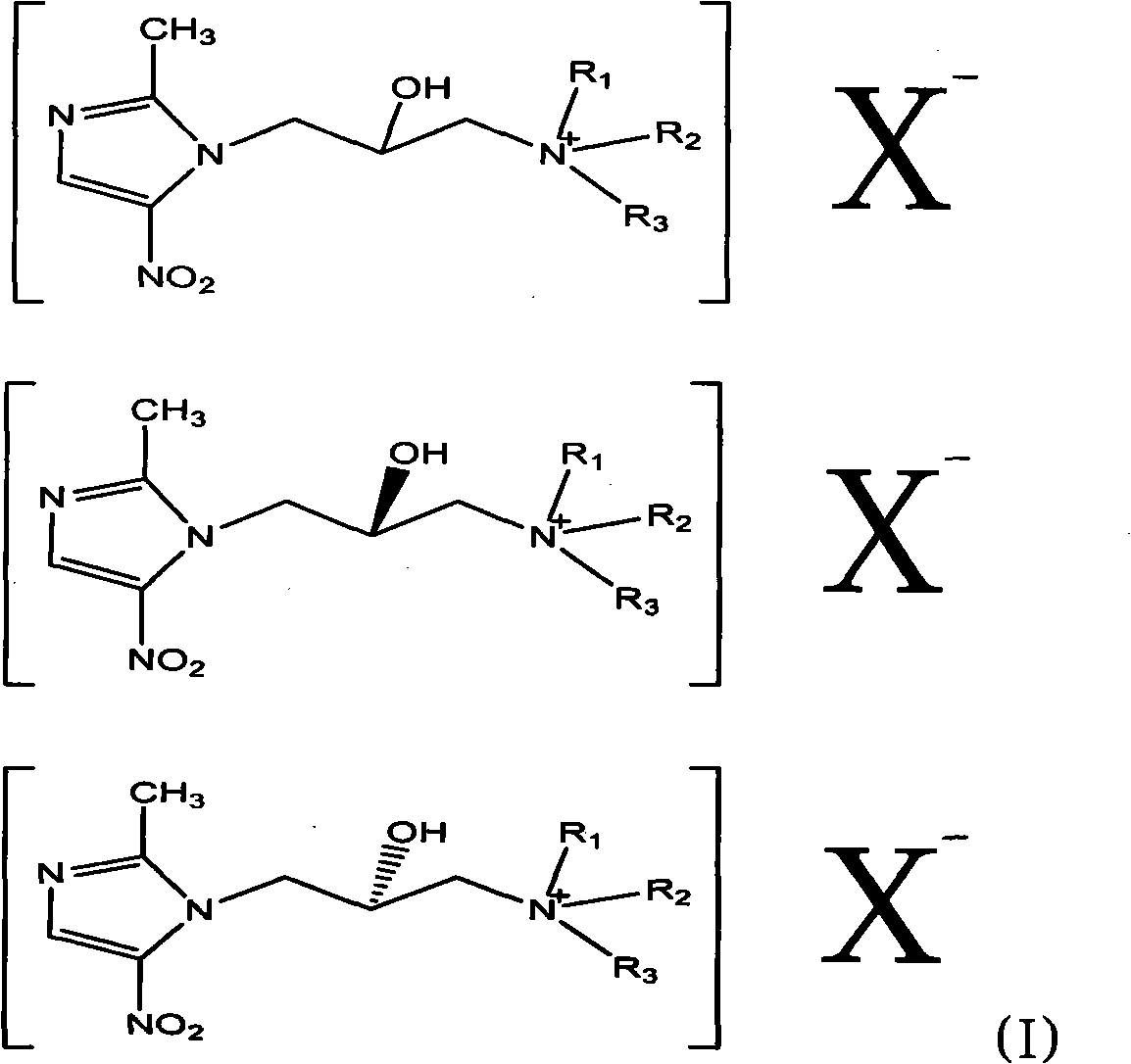
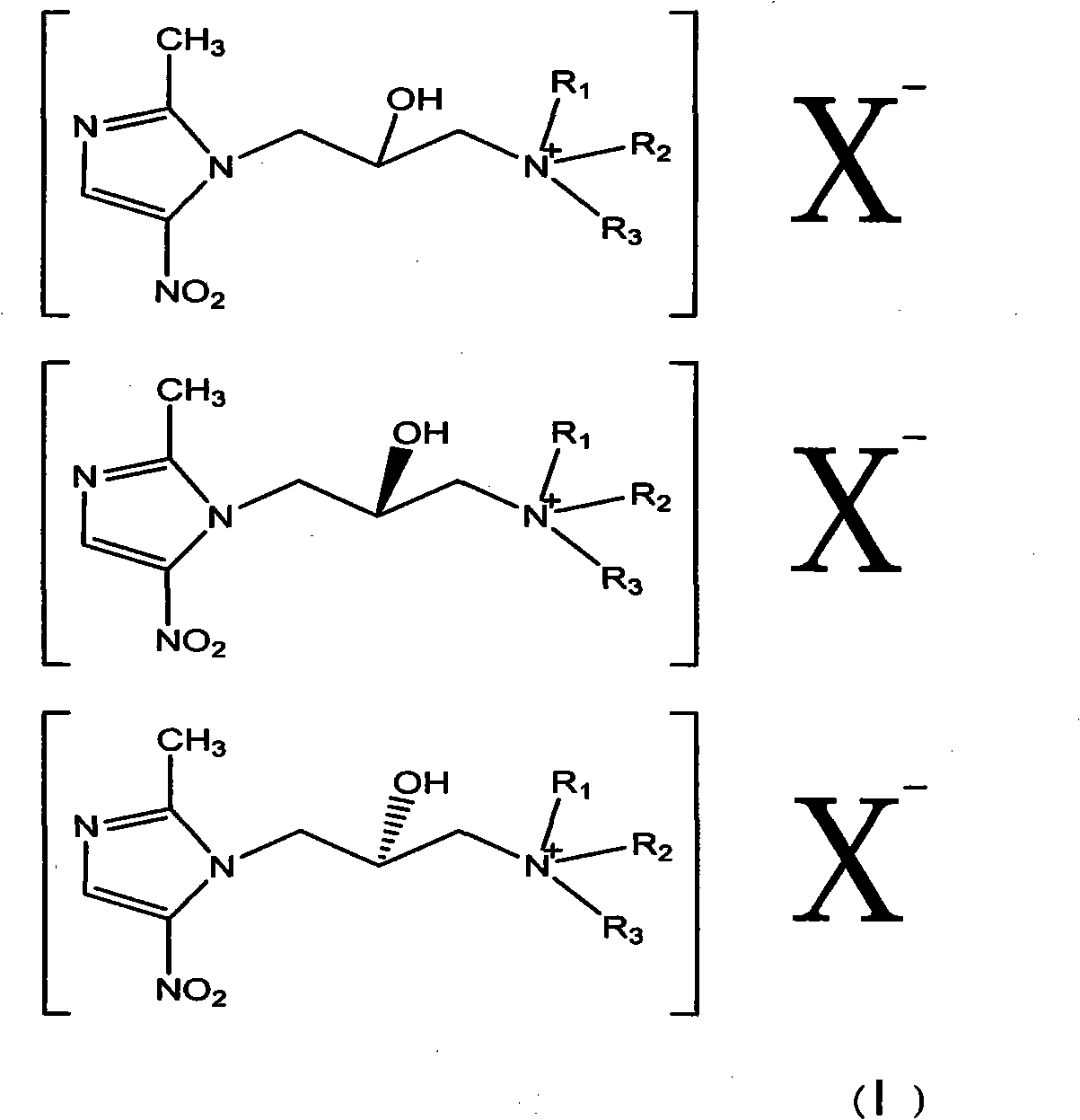
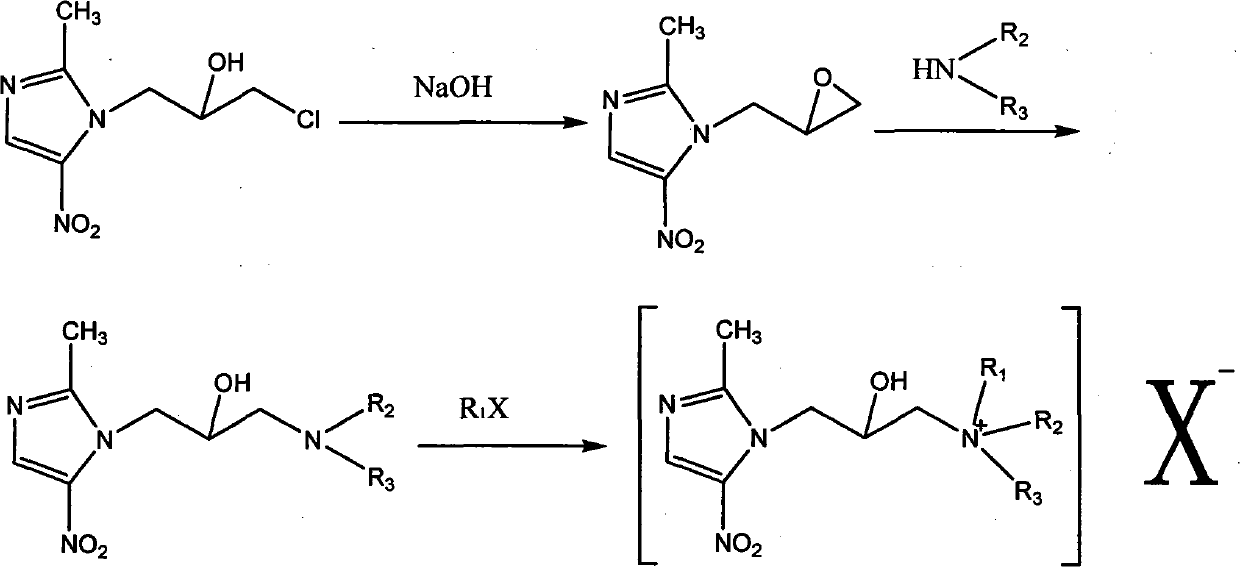
Nitroimidazole derivative in therapy
An alkyl and compound technology, applied in the field of nitroimidazole derivatives and pharmaceutical compositions thereof, can solve the problems of discomfort, autoimmune hepatitis, peripheral neuropathy, low water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of compound A
[0053]Dissolve 220g of 1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole (ornidazole) in 500ml of dichloromethane and slowly add 20% sodium hydroxide 250ML dropwise , stirred and reacted at room temperature for 40 minutes, separated, dried the organic layer, evaporated the solvent under reduced pressure, and the residue was 151g; slowly added 70g of pyrrolidine to the residue in 500ML ethanol solution, reacted at 50°C for 6 hours, terminated the reaction, evaporated under reduced pressure The solution was dried, and the residue was recrystallized with ethanol / ethyl acetate to obtain 92 g of a solid; 92 g of the solid was dissolved in 500 ml of methanol, and 55 g of methyl iodide was added dropwise with stirring, reacted at room temperature until the raw material point disappeared, and evaporated to dryness under reduced pressure to obtain 140 g of compound A.
[0054] Purity: 98.3% Chromatographic conditions C18 chromat...
Embodiment 2
[0063] Example 2: Preparation of Compound B.
[0064] Dissolve 220g of 1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole in 500ml of dichloromethane, slowly add 20% sodium hydroxide 250ML dropwise, and stir at room temperature for 40 Minutes, liquid separation, drying of the organic layer, evaporation of solvent under reduced pressure, residue 151g; residue with 500ML ethanol solution, slowly added morpholine 80g, 50 ° C for 10 hours, terminated reaction, solution under reduced pressure, residue Recrystallize with ethyl acetate to obtain 53g of solid; dissolve 53g of solid in 500ml of methanol, add 30g of iodomethane dropwise under stirring, react at room temperature until the raw material point disappears, and evaporate to dryness under reduced pressure to obtain 82g of compound A.
[0065] Purity: 98.1% Chromatographic conditions C18 chromatographic column (250mm×46mm, 5μm), mobile phase is acetonitrile-water (40:60), column temperature is 30°C, detection wavelength is ...
Embodiment 3
[0068] Example 3: Preparation of Compound C.
[0069] Dissolve 220g of 1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole in 500ml of dichloromethane, slowly add 20% sodium hydroxide 250ML dropwise, and stir at room temperature for 40 Minutes, liquid separation, drying of the organic layer, evaporation of solvent under reduced pressure, residue 151g; residue with 500ML ethanol solution, slowly added morpholine 80g, 50 ° C for 10 hours, terminated reaction, solution under reduced pressure, residue Recrystallize with ethyl acetate to obtain 53g of solid; dissolve 53g of solid in 500ml of methanol, add 45g of bromopropane dropwise under stirring, react at room temperature until the raw material point disappears, and evaporate to dryness under reduced pressure to obtain 87g of compound C.
[0070] Purity: 97.5% Chromatographic conditions C18 chromatographic column (250mm×46mm, 5μm), mobile phase is acetonitrile-water (40:60), column temperature is 30°C, detection wavelength is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com