Yeast expression system for expressing HAS-Vmip-II fusion protein and construction method thereof
An expression system and fusion protein technology, applied in the field of bioengineering, can solve problems such as toxic side effects, increase patient pain and treatment costs, blood drug concentration cannot inhibit virus replication, etc., and achieve the effect of simplifying the screening process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
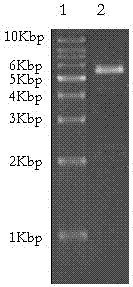
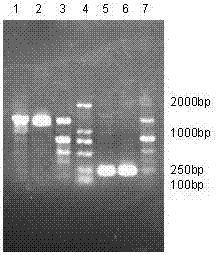
[0038] Example 1 Construction of HAS-vMIP-II Yeast Expression System
[0039] 1. Alkaline lysis method to extract the plasmid containing the target gene from Escherichia coli
[0040] (1) Cultivate Escherichia coli DH5a transformed with the recombinant plasmid pPICZaA-HSA-vMIP-II, and harvest the bacteria: Pour 1.5ml of bacterial liquid into a microcentrifuge tube, centrifuge at 12000g for 30 seconds, and absorb the culture medium. The supernatant must be aspirated clean, otherwise it will affect the quality of the plasmid. Centrifuge twice if necessary.
[0041] (2) Resuspend the bacterial pellet in 100 μl ice-cooled solution I (solution I preparation: 50 mM glucose / 25 mM Tris-HCl / 10 mM EDTA, pH 8.0; store at 4°C after autoclaving) , add 1 / 50 volume of RNase A stock solution, shake vigorously to make it completely dispersed. Glucose can be replaced by NaCl to facilitate storage; to extract plasmids larger than 15kb, the bacteria should be suspended in isotonic sucrose solu...
Embodiment 3
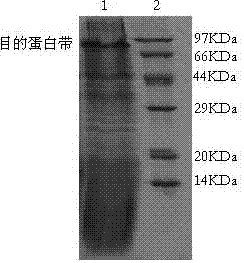
[0102] Example 3 Detection of biological activity of purified protein (chemotaxis inhibition experiment)
[0103] Preparation of PBMC: Take about 4ml of lymphocyte separation solution in a clean centrifuge tube, dilute 4ml of fresh healthy peripheral blood with an equal volume of RPMI1640 solution, carefully add to the lymphocyte separation solution, room temperature 1300 rpm, 12 min ;The solution will be divided into 3 layers, the middle layer of flocculent is the peripheral blood mononuclear cells, take the lymphocyte layer into another clean centrifuge tube, add 4~5ml RPMI1640 solution to wash, 1200 rpm, 8 min; Clear, re-suspend with 4ml RPMI1640 solution (containing 10% calf serum), and calculate the cell density on a cell counting plate.
[0104] Calculate the amount of diluent according to the adjusted cell density to adjust the cell density to 1×10 6 / ml, and resuspended in RPMI1640 medium (containing 10% calf serum).
[0105] The above cell suspension was treated wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| capacitance | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com