Feruloyl esterase A mutant and purpose thereof
A technology of ferulic acid esterase and mutants, which can be used in the fields of genetic engineering and enzyme engineering, and can solve problems such as unsatisfactory thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
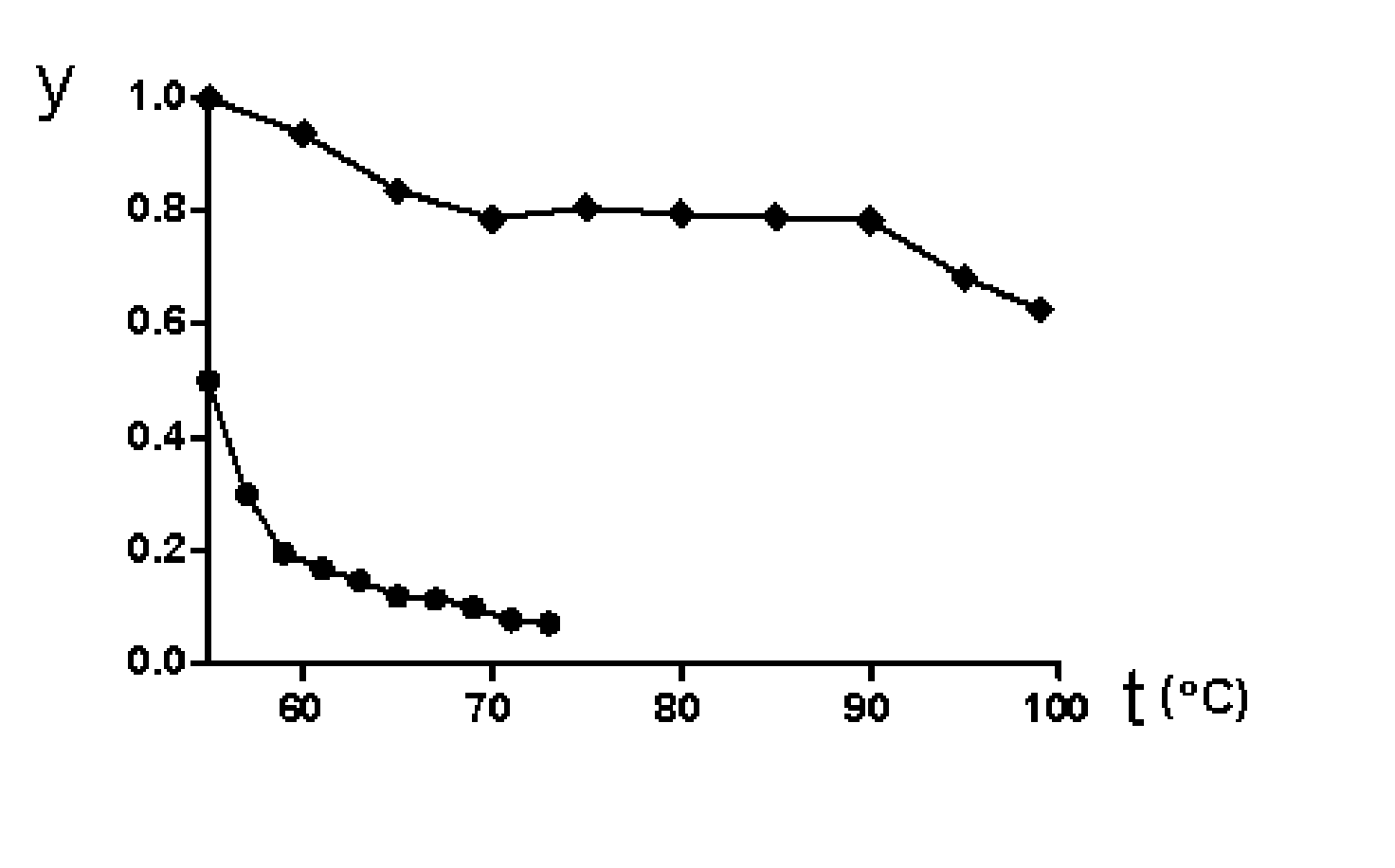
Image
Examples
Embodiment 1
[0024] Embodiment 1 Error-prone PCR (error-prone PCR) method constructs ferulic acid esterase library
[0025] use II Random Mutagenesis kit randomly mutates the ferulic acid esterase gene (see SEQ ID NO.1).
[0026] The primers used were: faeA-F: 5'-taggaggt gaattc gcctccacgcaaggcatctc-3'
[0027] faeA-R: 5'-taggaggt gcggccgc ttaccaagtacaagctccgctcg-3'
[0028] The reaction conditions were: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 61°C for 1 min and extension at 72°C for 1 min, a total of 25 cycles, and the gene fragments were recovered after electrophoresis.
[0029] The recovered fragment was double digested with NotI and EcoRI, and ligated with the pGAPZαA vector (containing the bleomycin resistance gene) that had undergone the same digestion. The molar ratio of pGAPZαA carrier digested by double enzyme digestion and the target gene fragment was mixed at a ratio of 1:3, and 600 units of T 4 Ligase, ligated overnight at 16°C. The...
Embodiment 2
[0030] The screening of embodiment 2 ferulic acid esterase mutant libraries
[0031] After collecting the secondary mutant library clones in Example 1, the plasmids were extracted and linearized with PmeI endonuclease, then electroporated to transform Pichia pastoris (Pichia pastoris KM71) competent cells and spread on MD plates (1.34% YNB, 4×10 -5% biotin, 2% glucose). After culturing for 2 days, single clones were picked and placed in a 96-well plate, each well containing 150 μl of BMGY medium (containing 100 mM, potassium phosphate buffer with a pH value of 6.0, 2% tryptone, 1% yeast extract, 1% glycerol, 4×10 -5 % biotin), 30°C, 280rpm, shake culture for 2 days. Use a 96-well plate replicator to replicate each single clone on an MD solid medium plate, culture it at 30°C for 2 days, and store it in a refrigerator at 4°C. After centrifuging the 96-well plate culture, remove the supernatant, and add 150 μl of BMMY (in addition to replacing 1% glycerol with 0.5% methanol in ...
Embodiment 3
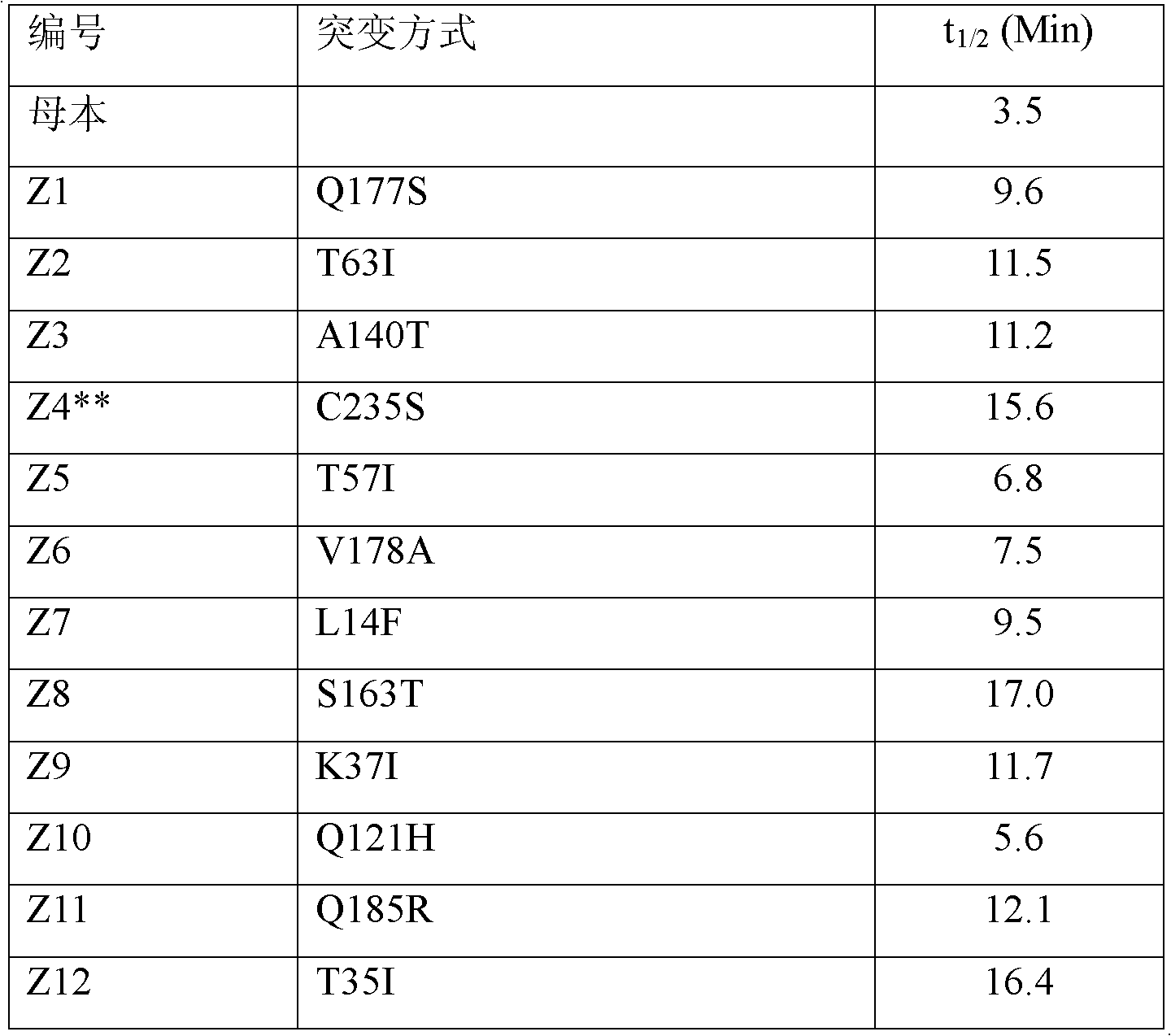
[0032] Embodiment 3 Integration of ferulic acid esterase mutation site
[0033] The 12 mutation sites obtained above were integrated into a nucleotide coding sequence and the complete sequence was synthesized by Shanghai Sangon Biotechnology Service Co., Ltd. In order to facilitate subsequent protein purification, the synthesized full sequence was double digested with NotI and EcoRI to recover the target fragment, and ligated into the pGAPZαA vector (containing the bleomycin resistance gene) after the same digestion (the ligation method is as in Example 1) . The ligated product was transformed into Escherichia coli DH5α, and the transformant was picked and cultured to extract the recombinant plasmid. After the plasmid was linearized with BspHI endonuclease, it was transformed into Pichia pastoris (Pichia pastoris KM71) competent cells, and the YPDS medium containing 100 μg / ml bleomycin (containing 2% tryptone, 1% yeast extract material, 2% glucose, 1M sorbitol), and cultured...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com