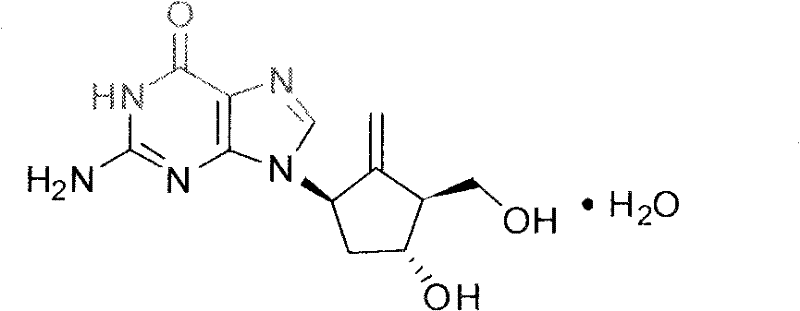
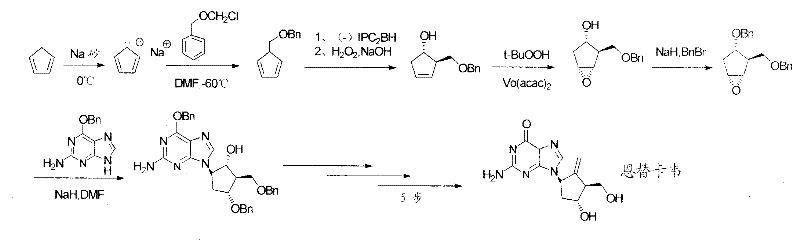
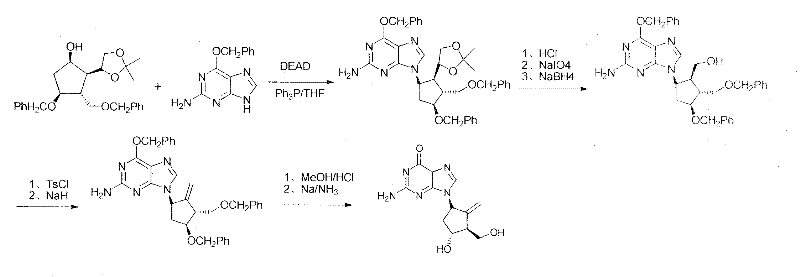
Novel synthesis process of entecavir monohydrate
A technology of monohydrate and entecavir, which is applied in the field of new synthesis technology of entecavir monohydrate, can solve the problems of low yield of entecavir monohydrate, unsuitable for industrial production, extremely high equipment requirements, etc., and achieves low price, easy control and reaction. selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of 1
[0060] The three-necked flask of preparing 10L is furnished with the atmospheric distillation device of short fractionation column (filler can be added), accepts the anhydrous calcium chloride of 50 grams in the bottle, and is placed in ice-water bath (monomer cyclopentadiene room temperature polymerization, It must be stored at low temperature), and the tail pipe has a drying tower. Add 1500 grams of dicyclopentadiene to the three-necked flask, protect the whole system with a slight nitrogen atmosphere, and slowly raise the temperature to 180°C under stirring, the monomer cyclopentadiene distills out in the receiving bottle, and keep the temperature of the upper gas port of the distillation not exceeding 42°C Finally, about 1200 grams of monomer cyclopentadiene (preserved at low temperature) were obtained.
Embodiment 2
[0061] Embodiment 2: Preparation of 2
[0062] Add 1000ml of anhydrous xylene, 400g of deoxidized surface metal sodium to a 5L three-necked flask in an oil bath, protect it with a little nitrogen, heat up to 120°C under stirring, dissolve the sodium, and stir vigorously to disperse the sodium into sodium sand. Stop stirring, bring the system back to room temperature, solidify the sodium sand, remove the xylene on the surface, replace it with an appropriate amount of anhydrous THF three times, and finally add about 6000ml of anhydrous THF for protection and set aside.
[0063] Under the protection of slight nitrogen, use an ice-water bath to cool the tetrahydrofuran-sodium sand to 0-5°C, slowly add the prepared monomer cyclopentadiene to the tetrahydrofuran-sodium sand system, control the temperature not to exceed 10°C, and drop After that, the ice-water bath was removed, and the temperature was naturally raised to room temperature and stirred for about 3 hours. The sodium sand...
Embodiment 3
[0064] Embodiment 3: Preparation of 3
[0065] Add 2,500 grams of dimethylphenylchlorosilane and 6,000 ml of anhydrous tetrahydrofuran into a 10L reaction kettle, under the protection of a slight nitrogen gas, cool the system to below -70°C, start adding 2 dropwise, and control the dropping temperature below -70°C, After the dropwise addition, keep stirring at -70°C for about 3 hours, TLC detects that the reaction is complete, (developing agent n-hexane), let it naturally warm up to 0°C, slowly add ice water, stir, stand and separate, and use for the organic phase Wash with saturated sodium bicarbonate solution, extract with n-hexane, dry over anhydrous sodium sulfate, and concentrate under reduced pressure at 65°C to finally obtain about 2700 g of dark yellow oil 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com