Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) fusion protein, its preparation and applications
A technology of fusion protein and protein, which is applied in the direction of antineoplastic drugs, peptide/protein components, biochemical equipment and methods, etc., and can solve the problems of short half-life and affecting the efficacy of TRAIL protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1. Construction of fusion protein expression vector
[0056] Using the leucine zipper (LZ, including cross-linking region and human leucine zipper domain) fragment synthesized by Shanghai Xuguan Biotechnology Development Co., Ltd. as a template, the required DNA fragment was amplified by PCR method. The primers used LZ1 and LZ2 were synthesized by Shanghai Sangon Bioengineering Technology Co., Ltd. Wherein the primer LZ2 not only contains the 3' end sequence of the LZ fragment, but also adds a sequence encoding a GlySer linker peptide after its 3' end.
[0057] LZ1: 5'CATGCCATGGCCCATATGGAGGAAGACCCGTGTGC
[0058] LZ2: 5'CGGGATCCGACAACTGTGTTTCCAGGATG
[0059] The TRAIL fragment is the 95th to 281st amino acid of the complete sequence of TRAIL, which is amplified from the cDNA library of human fetal liver bank (purchased from Clontech Lanoratories Inc.USA) by PCR method, and the primers TRAIL1 and TRAIL2 used are provided by Shanghai Sangong Bioengineering Techno...
Embodiment 2
[0064] Embodiment 2: Preparation of fusion protein
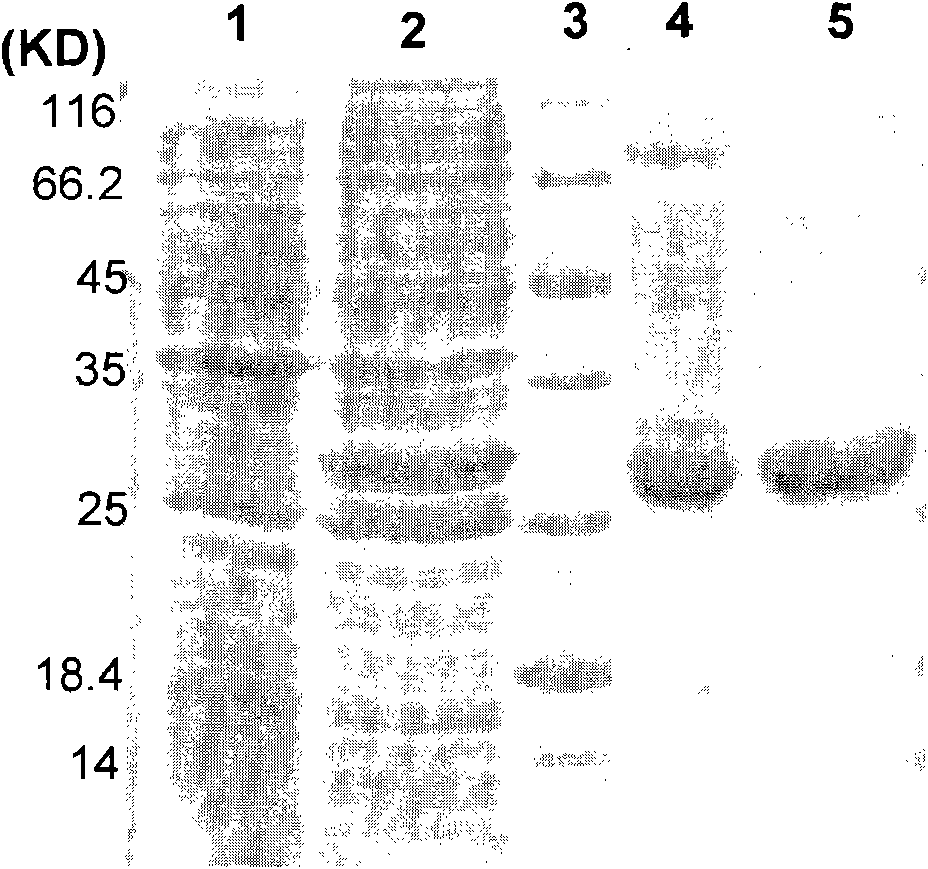
[0065] The Escherichia coli Rosetta clone transformed with the recombinant plasmid pET-LZ-TRAIL was cultured in a culture flask containing LB medium (37°C), and when the bacterial density reached OD600≈0.8, 1mM isopropyl-β- D-thiogalactoside (IPTG) induced expression. After about 6 hours, the bacterial cells were harvested at 5,000×g (30 minutes).
[0066] The harvested bacteria were resuspended in Tris buffer (pH 8.0), and the bacteria were disrupted by ultrasonication. After breaking the bacteria, centrifuge at 10,000×g for 30 minutes to collect the supernatant. The crushed supernatant obtained by the above method was applied to Ni sepharose 6 Fast flow (GE Company) for metal affinity chromatography, 40 mM imidazole was used to elute the impurity protein, and then 120 mM imidazole was used to elute the recombinant protein. The eluate was further purified by ion-exchange chromatography using Qsepharose Fast flow (GE Comp...
Embodiment 3
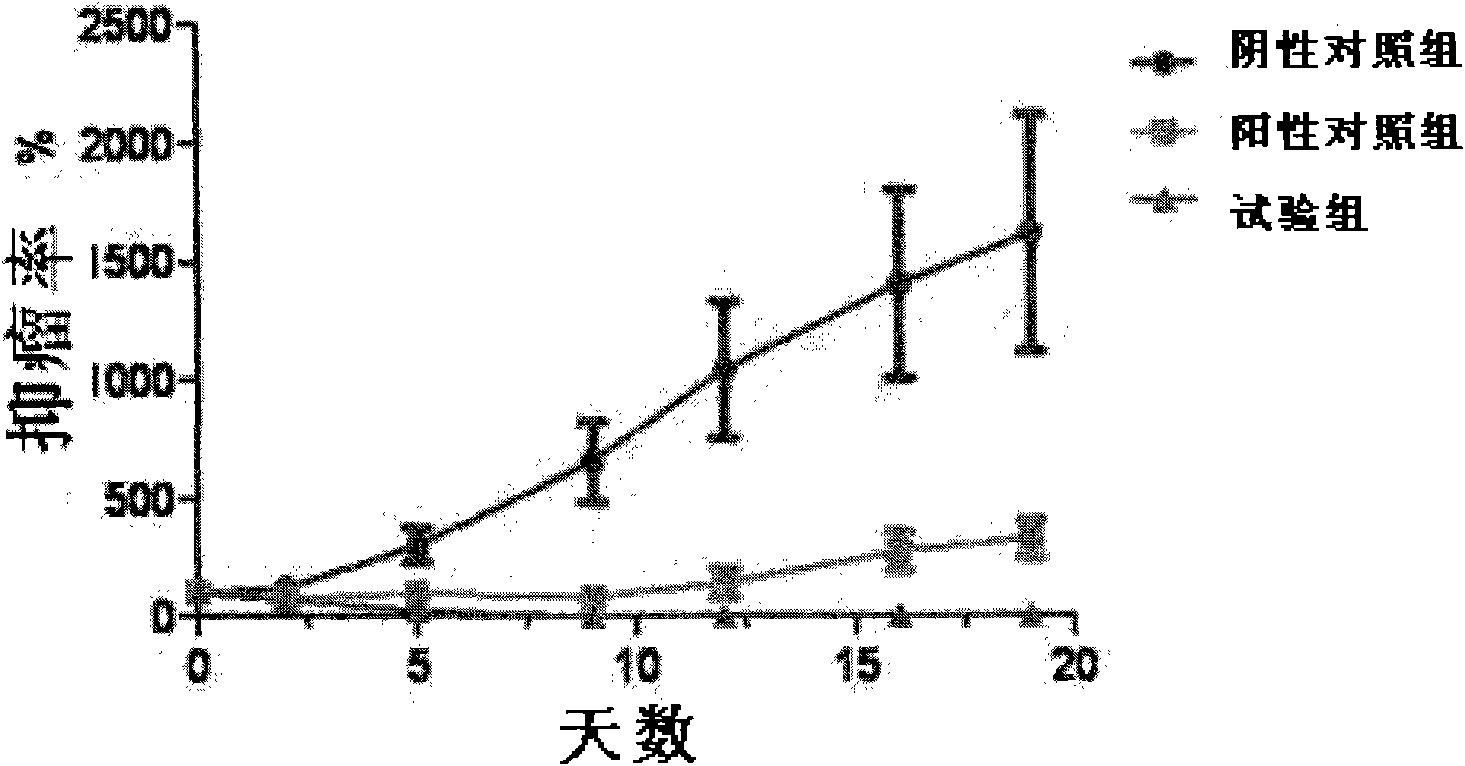
[0067] Embodiment 3: Drug efficacy experiment in vivo
[0068] Breast cancer cells MDA-MB231 were inoculated subcutaneously to establish a tumor-bearing nude mouse model, and experiments were carried out after the model was established. The experiment was divided into a negative control group, a positive control group (the extracellular region of the human TRAIL protein, the amino acid sequence of which is shown in SEQ ID NO: 6) and a test group (LZ-TRAIL). 6 nude mice in each group, the tumor tissue was inoculated to grow to 0.1cm3 and administered subcutaneously every day, according to equimolar dosage, the dosage was 300ug / mouse / time (TRAIL) and 450ug / mouse / time (LZ-TRAIL) respectively. ), administered 14 times in total. The nude mice were observed for 6 days after drug withdrawal, the tumors were peeled off and weighed, and the average tumor type and tumor inhibition rate of each group were calculated. The tumor inhibition rate was calculated by the following formula.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com