Medical component and manufacturing method thereof
A manufacturing method and medical technology used in pharmaceutical formulations, pharmaceutical sciences, bone implants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
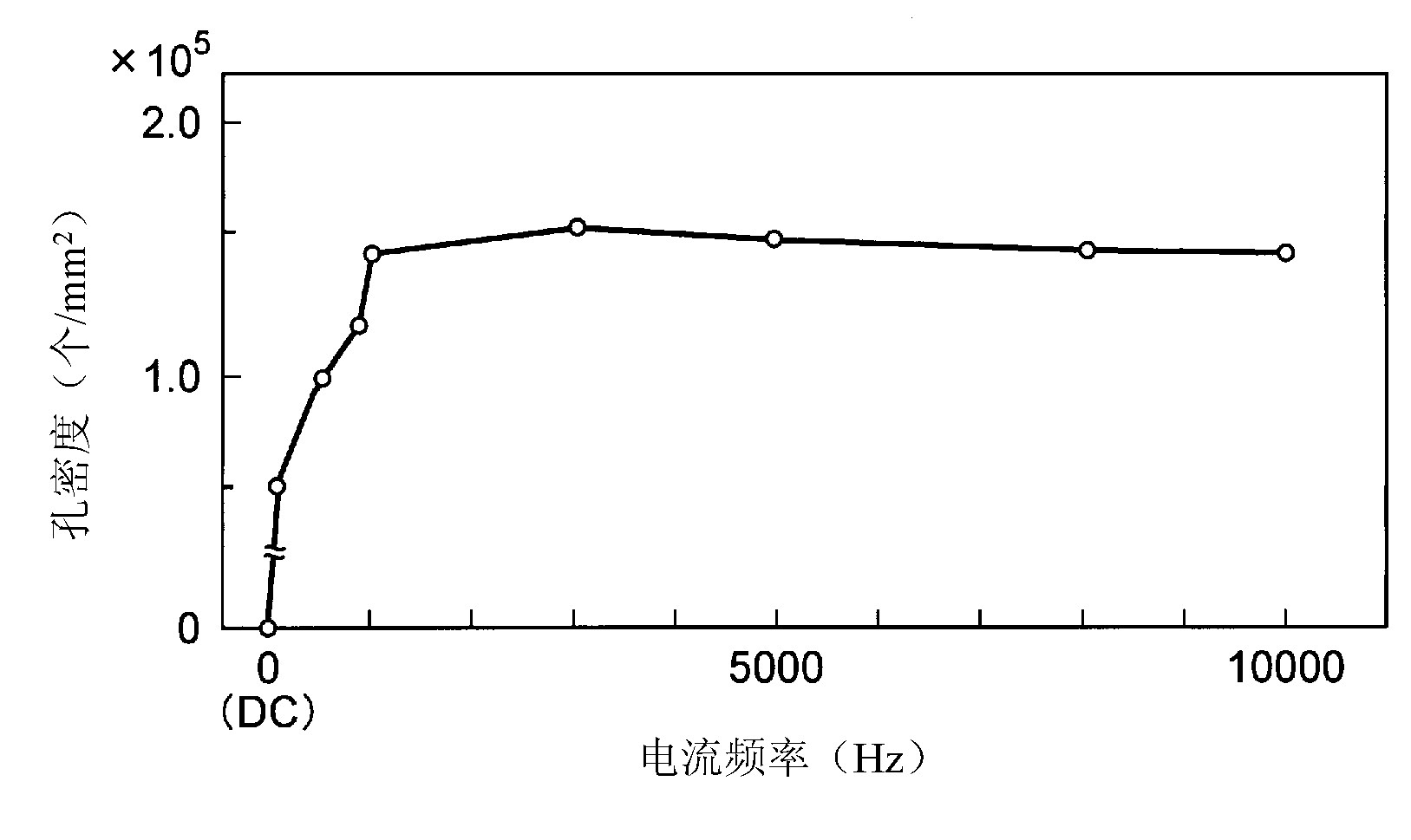
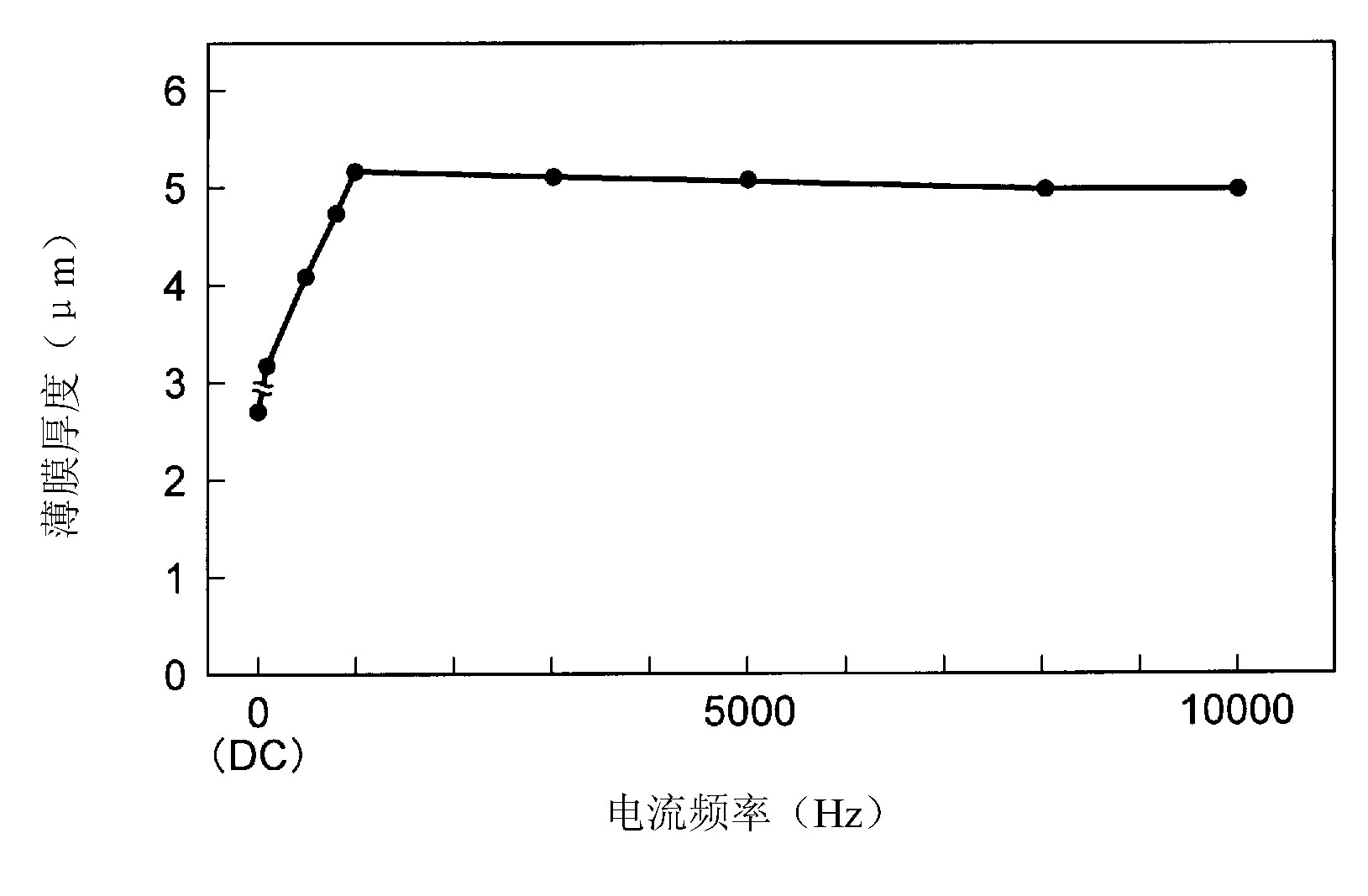
[0072] Using Ti alloy (mass%, 6%Al-4%V-the rest of Ti, JIS 60 alloys), stainless steel (SUS304) as the base material, made a disc (thickness: 2.0mm) as an in vitro (in vitro ) experimental test piece, made of external bone fixation needles for rabbits as a test piece for in vivo experiments. In addition, among these test pieces, the test piece made of Ti alloy was degreased and then anodized. Anodizing treatment is carried out in an acidic electrolyte (liquid temperature: room temperature) in a mixed pool of sulfuric acid (35g / l)-phosphoric acid (25g / l)-hydrogen peroxide (10g / l), electrolysis at a constant voltage (150V) for 5 minutes processing. The initial current density of the applied current is 8A / dm 2 , due to constant voltage electrolysis, the current value gradually decreases with time. In addition, the electric current is a pulse current with a frequency of 1000 Hz. The case where anodic oxidation treatment was not performed was taken as a comparative example.
...
Embodiment 2)
[0087]Using stainless steel (SUS 304) as the substrate, prepare disc test pieces (thickness: 2mm) for antibacterial tests. These test pieces were subjected to pickling and cleaning treatment, and then anodized treatment or chemical treatment. The pickling treatment is immersion in a mixed aqueous solution of nitric acid (5%)-hydrofluoric acid (3%) at a liquid temperature of 40°C for 3 minutes. In addition, the anodizing treatment is carried out in an acidic electrolyte solution (liquid temperature: room temperature) in a hydrochloric acid (47% by mass)-hydrogen peroxide (2% by mass)-formalin (10% by mass)-water mixing pool. The test piece was used as an anode, and the pure Ti plate was used as a cathode, and electrolysis was performed at a constant voltage (100V) for 15 minutes. In addition, the applied current was a pulse-like current with a frequency of 3000 Hz. The initial current value is 3.5A / dm 2 . The chemical treatment was a process of immersing for 30 minutes in a...
Embodiment 3)
[0099] Using Co-Cr alloy (mass %: 63.0%Co—6.0%Mo—2.0%Ni—0.25%C—the rest of Cr) as the base material, make a disc test piece for antibacterial test (plate thickness: 5.0mm) . These test pieces were subjected to pickling and cleaning treatment, followed by anodizing treatment.
[0100] Anodizing treatment is potassium hydroxide (165g / l) - potassium fluoride (35g / l) - sodium phosphate (35g / l) - aluminum hydroxide (35g / l) - alkaline electrolyte of water mixing pool (liquid temperature : room temperature), the test piece was used as the anode, and the pure Ti plate was used as the cathode, and electrolytic treatment was performed for 15 minutes at a DC constant voltage (150V). In addition, the applied current was a pulsed current with a frequency of 5000 Hz. The initial current value is 8A / dm 2 .
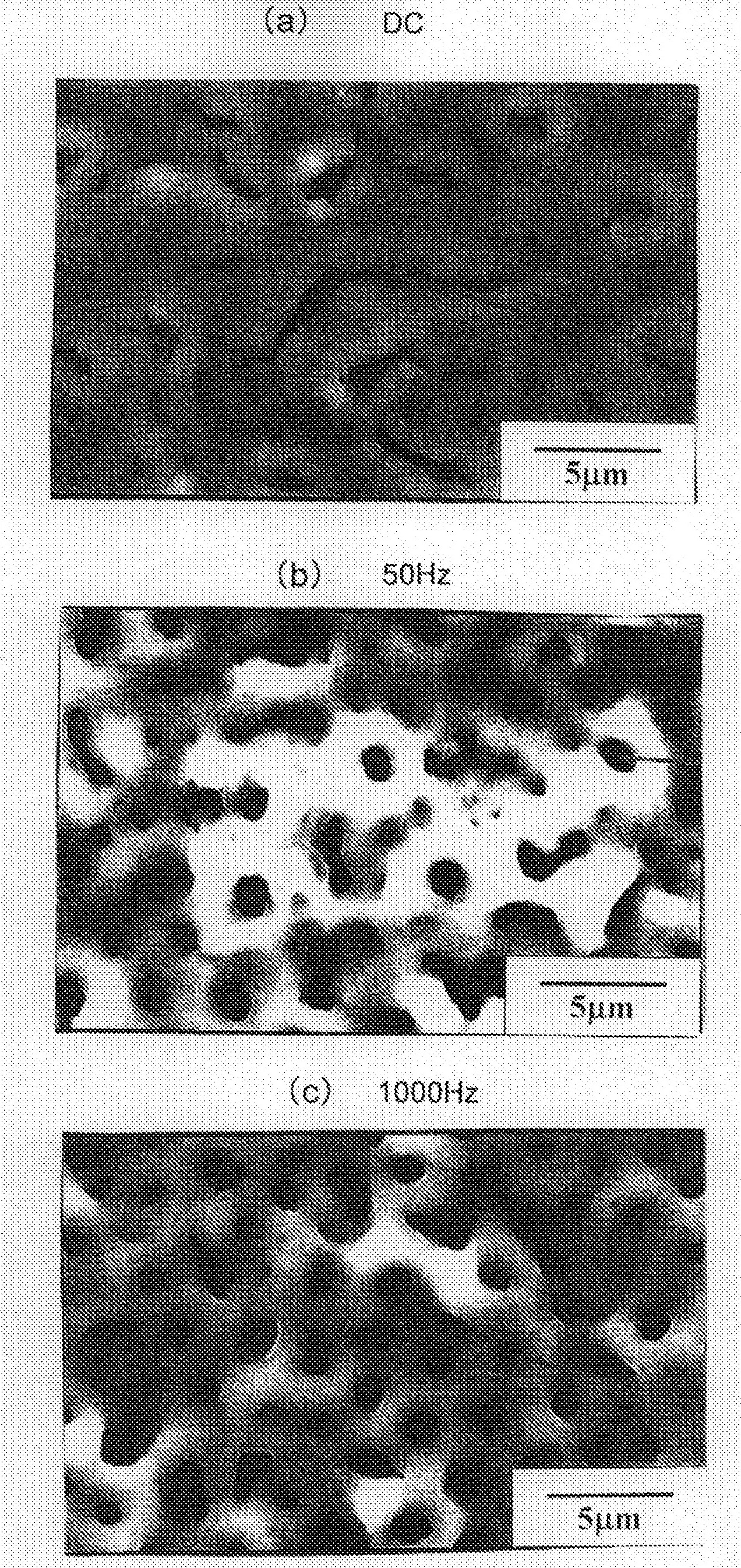
[0101] For the obtained test pieces, in the same manner as in Example 2, the surface conditions of the thin films (oxidized films) formed on the surfaces of each test pieces were obs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com