Preparation method of porcine parvovirus genetic engineering vaccine
A technology of genetically engineered vaccine and parvovirus, which is applied in the field of preparation of porcine parvovirus genetically engineered vaccine, can solve the problems of loose virus, difficult to popularize and apply, and short immunity period, so as to prevent degradation, ensure biological activity, and be easy to separate The effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Construction of recombinant yeast expression vector: the expression vector is pGAPZαA, and the host strain is yeast strain SMD1168. The whole gene sequence of PPV VP2 was amplified by PCR, the upstream primer P1 was shown in SEQ ID NO:1, the downstream primer P2 was shown in SEQ ID NO:2, and the enzyme cutting sites were XhoI and NotI, AAAAGA It is the Kex2 protease cleavage site.
[0025]2. Construction of recombinant yeast engineering bacteria: Transform yeast strain SMD1168 by linearization electroporation with the recombinant yeast expression vector constructed above, and the linearization enzyme is AvrⅡ.
[0026] 3. Non-induced expression of recombinant yeast engineered bacteria: Pick the single Zeocin-resistant colony screened with a sterilized pipette tip, and carry out primary culture in 5mL YPD liquid medium, shake overnight at 30°C and 200 r / min , to OD 600 =2-6, that is, the cells are in the logarithmic growth phase. Take 1mL of primary culture solution...
Embodiment 2
[0030] Animal experiment: The PPV genetic engineering vaccine prepared above was used to immunize piglets with basically no difference in body weight and good mental status and PPV negative. At the same time, a positive control, an empty vector control, a normal saline control, an adjuvant control and a blank control without any treatment were set up. Immunization was boosted 15 days later, and after antibodies were detected by ELISA kits, a virulent strain of porcine parvovirus was used for challenge experiments (except group F) to detect whether the antibodies produced had protective power. The experiment is as follows:
[0031] test group Inoculum dose Group A PPV Genetic Engineering Vaccine 3 servings Group B Positive control (PPV attenuated vaccine) 3 servings Group C Empty vector control 3 servings Group D saline control 3 servings Group E Adjuvant control 3 servings Group F Blank control without any treatm...
Embodiment 3
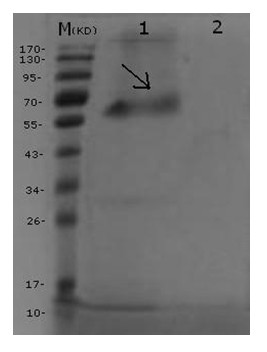
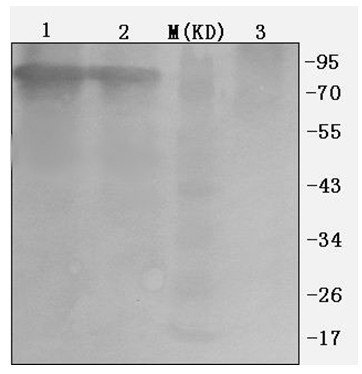
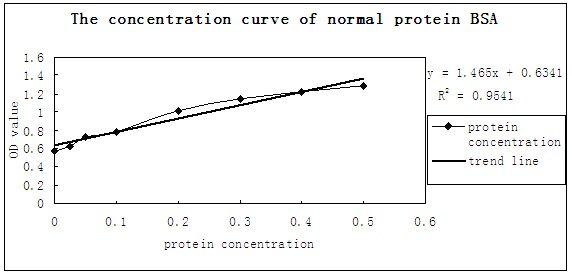
[0034] PPV VP2 expression measurement: VP2 expression supernatant protein concentration was determined using Bradford protein concentration determination kit. Since there is a small amount of bacterial protein in the expression supernatant, the measured concentration is the total protein concentration. The specific steps are as follows:
[0035] 1. Completely dissolve protein standard BSA (5 mg / mL), take 10 μL and dilute to 100 μL, so that the final concentration is 0.5 mg / mL. Standards were diluted with PBS.
[0036] 2. Add 0, 1, 2, 4, 8, 12, 16, 20 μL of the diluted standard to the standard wells of the 96-well plate, and add PBS diluent to make up to 20 μL.
[0037] 3. Add 10 μL of the supernatant of VP2 expression time of 48h, 72h, and 96h to the sample wells of the 96-well plate, and add PBS diluent to 20 μL.
[0038] 4. Add 200 μL of G250 staining solution to each well and let stand at room temperature for 3-5 minutes.
[0039] 5. Measure the absorbance at a wavelength...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com