Fresh animal medicinal composition and method for measuring content of Chinese medicines of fresh animal medicinal composition
A determination method and composition technology, which can be used in measurement devices, material separation, and analysis of materials, etc., and can solve problems such as lack of quality control research and poor specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
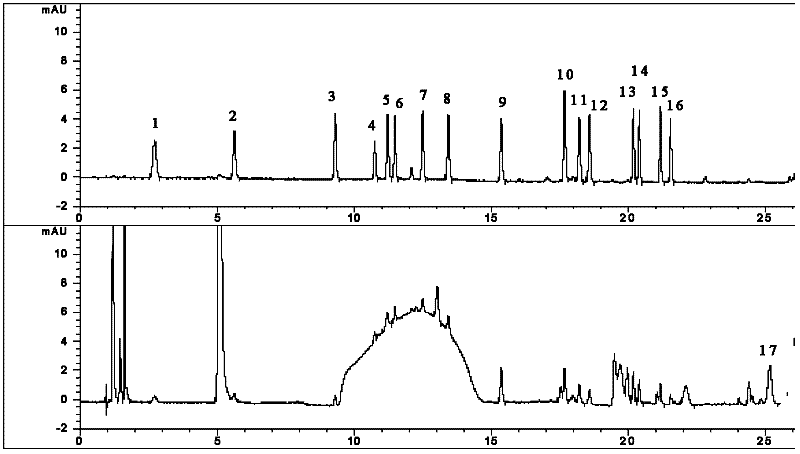
[0136] Experimental Example: Quantitative Analysis of Amino Acids in the Pharmaceutical Composition Jinlong Capsules of the Present Invention and Various Raw Materials
[0137] 1. Experimental materials and equipment
[0138] 1.1 Experimental materials: 17 amino acids (aspartic acid, glutamic acid, serine, histidine, glycine, threonine, arginine, alanine, tyrosine, cystine, valine, methionine , phenylalanine, isoleucine, leucine, lysine and proline) mixed standard solution, borate buffer solution, OPA and FMOC derivatization reagents were purchased from Agilent; methanol and acetonitrile were purchased from B&J Company Provided, chromatographically pure; sodium dihydrogen phosphate, hydrochloric acid and sodium hydroxide are all analytically pure, purchased from Beijing Chemical Plant; the pharmaceutical composition Jinlong Capsule of the present invention (prepared according to the method of Example 1) and the raw materials of the pharmaceutical composition of the present inv...
Embodiment 1
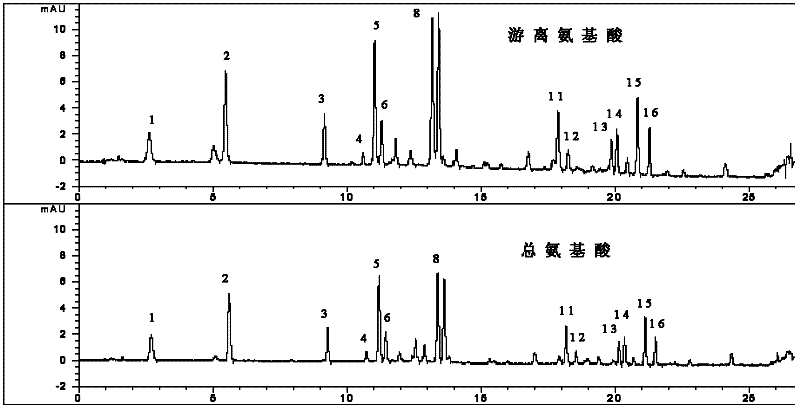
[0242] The assay method of embodiment 1 pharmaceutical composition capsule of the present invention
[0243] 1. Preparation of capsules
[0244] 1500kg of fresh Shougong, 750kg of fresh white snake and 750kg of fresh Viper, cut fresh Shougong, fresh white snake and fresh Viper into minced meat, grind it into a paste, add an equal amount of water to homogenate, and place Freeze at -25°C for 24 hours, take it out, put it in a 37°C water bath to melt, repeat three times, filter, the filtrate is centrifuged at 8000 rpm, absorb the supernatant, ultrafilter, take the filtrate and add 172kg of β-cyclodextrin for inclusion , the filtrate is vacuum freeze-dried, crushed into fine particles, dried at low temperature, and made into capsules;
[0245] 2. Content determination
[0246] (1) Preparation of standard solution:
[0247] a. 0.1mol / L hydrochloric acid solution: Take 0.83mL of 37.5% concentrated hydrochloric acid in a 100mL measuring bottle, dilute with water and set the volume...
Embodiment 2
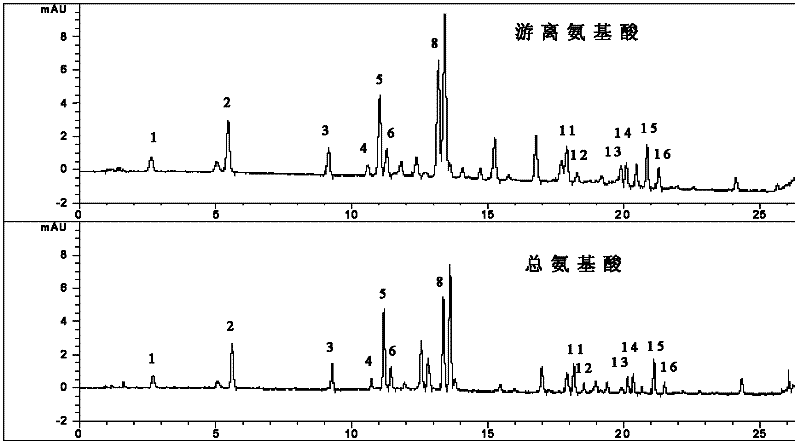
[0267] Embodiment 2 The method for assaying the content of the raw material drug of the pharmaceutical composition of the present invention, fresh snake snake
[0268] 1. Preparation of fresh Snake japonica powder
[0269] Take 3000g of fresh white snake, chop into minced meat, grind into a paste, add an equal amount of water to homogenate, freeze at -25°C for 24 hours, take it out, put it in a water bath at 37°C to melt, repeat three times, filter, The filtrate was centrifuged at 8000 rpm, the supernatant was absorbed, ultrafiltered, the filtrate was added with 172 g of β-cyclodextrin for inclusion, the filtrate was vacuum freeze-dried, crushed into fine particles, and dried at low temperature to make a powder.
[0270] 2. Content determination
[0271] (1) Preparation of standard solution: (same as Example 1)
[0272](2) preparation of need testing solution
[0273] a, the preparation of free amino acid need testing solution: get raw material drug of the present invention...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com