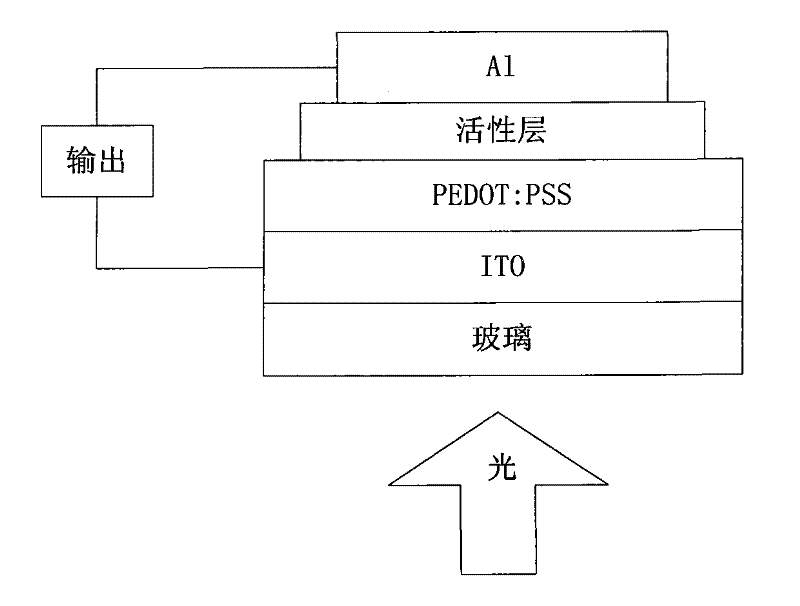
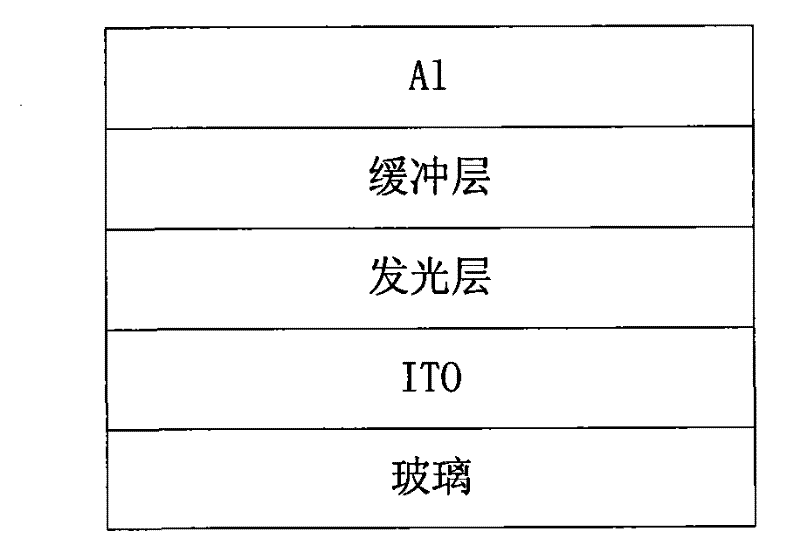
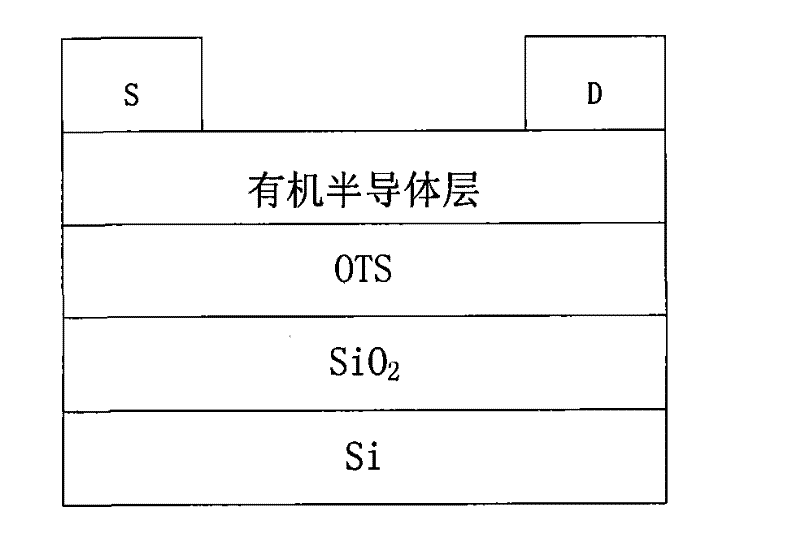
Perylenetetracarboxylic diimide copolymer containing thiophenepyrrole dione unit, preparation method thereof and application thereof
A technology of perylene tetracarboxylic acid diimide and pyrrole diketone, which is applied in the field of organic compound synthesis, can solve the problems of reducing the photoelectric conversion efficiency of organic solar cells, unable to use sunlight effectively, and the emission spectrum matching degree is not enough, etc. The effect of excellent charge transport performance, high yield, and excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a method for preparing the perylenetetracarboxylic diimide copolymer containing thienopyrrole diketopyrrole units, which comprises the following steps:
[0033] Step S1, respectively provide the compound I represented by the following structural formula 1 , I 2 ,
[0034]
[0035] Among them, R 1 , R 2 , R 3 selected from -H, C 1 ~C 20 Alkyl, C 1 ~C 20 Alkoxybenzene or phenyl; R 4 , R 5 from C 1 ~C 20 the alkyl group; R 6 from C 1 ~C 20 Alkyl or alkoxy;
[0036] Step S2, in an oxygen-free environment, in a system containing the first catalyst and the first organic solvent, compound I 1 , I 2 Carry out Stille coupling reaction, generate the described thienopyrrole diketone unit perylenetetracarboxylic diimide copolymer containing the following formula compound I,
[0037]
[0038] In the formula, n is an integer between 1 and 200.
[0039] The chemical reaction formula of the above-mentioned Stille coupling react...
Embodiment 1
[0065] Poly N,N'-bis-(3,4,5-tri-methylbenzene)-3,4,9,10-perylenediimide-5-methyl-1,3-bis(thiophene-2 -yl)-4H-thiophene[3,4-c]pyrrole-4,6(5H)-dione (n=11):
[0066] 1. Preparation of N, N'-two-(3,4,5-tri-methylbenzene)-1,7-dibromo-3,4,9,10-perylenediimide
[0067]
[0068] Weigh 0.27mmol 1,7-dibromo-3,4,9,10-perylene tetraanhydride in a reaction flask, add 0.84mmol 3,4,5-tri-methyl-1-aminobenzene and 12ml propionic acid , placed in an ultrasonic device for ultrasonication for 20 minutes, then heated to 80°C after 30 minutes of nitrogen gas, reacted for 48 hours, then cooled to room temperature, added chloroform to dissolve, and washed the organic layer with sodium bicarbonate solution to obtain The red suspension was filtered, dried by adding anhydrous magnesium sulfate, and spin-dried. The product was obtained after column separation (dichloromethane:petroleum ether=3:1). MS (EI) m / z: 784 (M+)
[0069] 2. Preparation of 5-methyl-1,3-bis(5-thiophen-2-yl)-4H-thiophene[3,4...
Embodiment 2
[0082] 1. N, N'-bis-(3,4,5-tri-methoxybenzene)-1,7-dibromo-3,4,9,10-perylenediimide and 5-octyl- For the preparation of 1,3-bis(5-tributyltinthiophen-2-yl)-4H-thiophene[3,4-c]pyrrole-4,6(5H)-dione, refer to Example 1;
[0083] 2. Poly N, N'-bis-(3,4,5-tri-methoxybenzene)-3,4,9,10-perylene diimide-5-octyl-1,3-bis( Preparation of thiophen-2-yl)-4H-thiophene[3,4-c]pyrrole-4,6(5H)-dione (n=14):
[0084]
[0085] Under the protection of argon, to the compound N,N'-bis-(3,4,5-tri-methoxybenzene)-1,7-dibromo-3,4,9,10-perylenediimide Amine 0.5mmol, 5-octyl-1,3-bis(5-tributyltinthiophen-2-yl)-4H-thiophene[3,4-c]pyrrole-4,6(5H)-dione 0.5mmol Dioxane (15 mL) solution. Introduce argon, bubble 0.5h to remove residual oxygen. Then add Pd(PPh 3 ) 2 Cl 2 10mg, continue to feed argon, bubble 0.5h to remove residual oxygen, and then heat to 85°C for 36 hours. The mixture was added dropwise to methanol for settling. Suction filtration, washing with methanol, and drying. It was then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com