Method for preparing ferrocenyl alkynyl porphyrin molecule possessing nonlinear optical activity and application thereof
A nonlinear optics, alkynyl porphyrin technology, applied in nonlinear optics, optics, organic chemistry, etc., can solve the problems of low nonlinear coefficient, inability to integrate semiconductor materials, etc., to achieve simple process, easy operation, high performance The effect of conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of 5,15-ferrocenyl-10,20-diphenylporphyrin
[0025] (1) Dissolve 5,15-dibromo-10,20-diphenylzinc porphyrin (305mg, 0.45mmol) and ferroceneacetylene (284mg, 1.35mmol) in tetrahydrofuran (10ml), stir and add di( Triphenylphosphine) palladium dichloride Pd(PPh 3 ) 2 Cl 2 (30mg, 0.04mmol), cuprous iodide CuI (3mg, 0.02mmol), ethylenediamine (2ml), reacted at 65°C for 10 hours, after the reaction was over, the solvent was removed under reduced pressure, and purified on a silica gel column Purified by washing with chloroform, and recrystallized from chloroform and methanol to obtain 5,15-ferrocenyl-10,20-diphenylzinc porphyrin with a yield of 60%.
[0026] (2) Dissolve 5,15-ferrocenyl-10,20-diphenylzinc porphyrin (200 mg, 0.12 mmol) in 20 mL of chloroform, add 5 mL of hydrochloric acid and stir for 1 h, then wash with distilled water, and separate the layers. After drying, the solvent was removed under reduced pressure to obtain the target product 5,15-ferroce...
Embodiment 2
[0028] Preparation of 5,15-ferrocenyl-10,20-diphenylporphyrin
[0029] (1) Dissolve 5,15-dibromo-10,20-diphenylzinc porphyrin (305mg, 0.45mmol) and ferroceneacetylene (378mg, 1.80mmol) in tetrahydrofuran (10ml), stir and add di( Triphenylphosphine) palladium dichloride Pd(PPh 3 ) 2 Cl 2 (20mg, 0.03mmol), cuprous iodide CuI (6mg, 0.04mmol), ethylenediamine (2ml), reacted at 55°C for 12 hours, after the reaction was over, the solvent was removed under reduced pressure, and purified on a silica gel column Chloroform was rinsed for purification, and recrystallized from chloroform and methanol to obtain 5,15-ferrocenyl-10,20-diphenylzinc porphyrin with a yield of 52%.
[0030] (2) Dissolve 5,15-ferrocenyl-10,20-diphenylzinc porphyrin (200 mg, 0.12 mmol) in 20 mL of chloroform, add 7 mL of hydrochloric acid and stir for 0.5 h, then wash with distilled water, and separate , dried, and the solvent was removed under reduced pressure to obtain the target product 5,15-ferrocenyl-10,2...
Embodiment 3
[0032] Determination of third-order nonlinear properties of 5,15-ferrocenyl-10,20-diphenylporphyrin
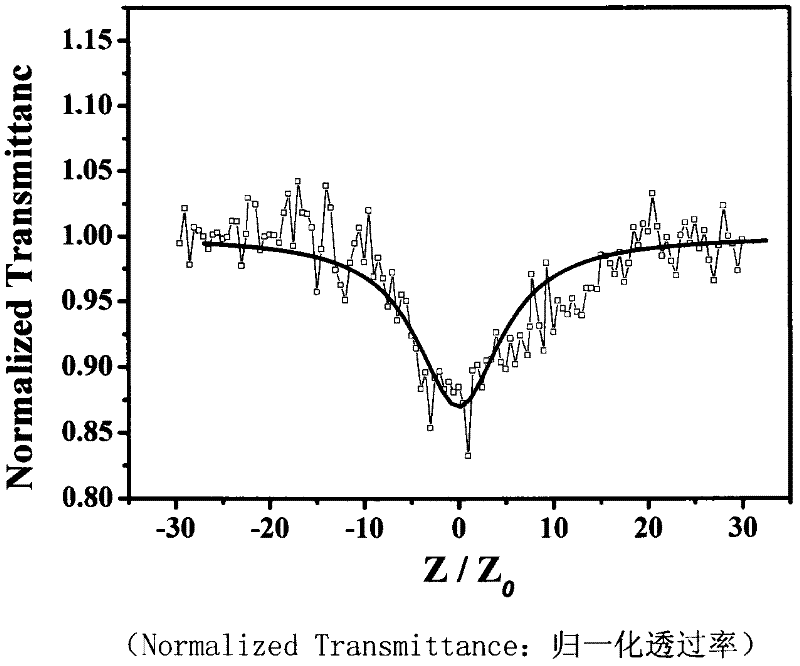
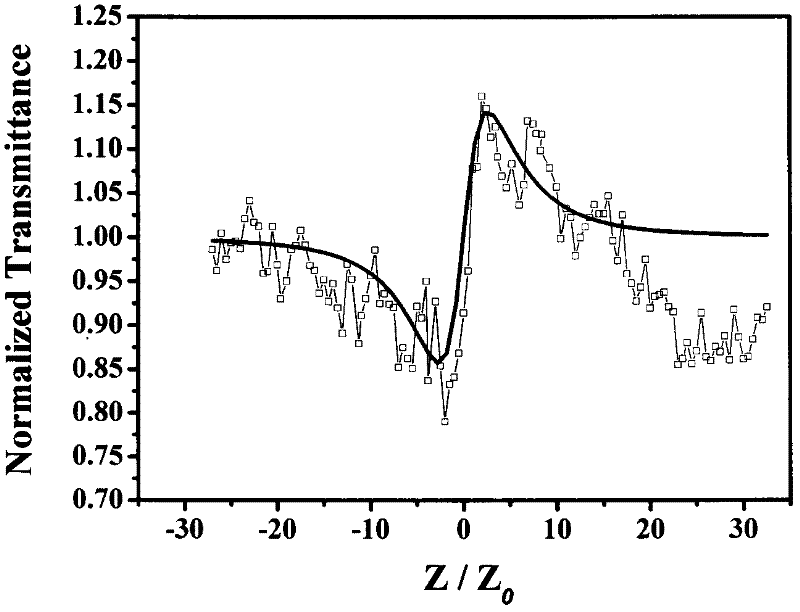
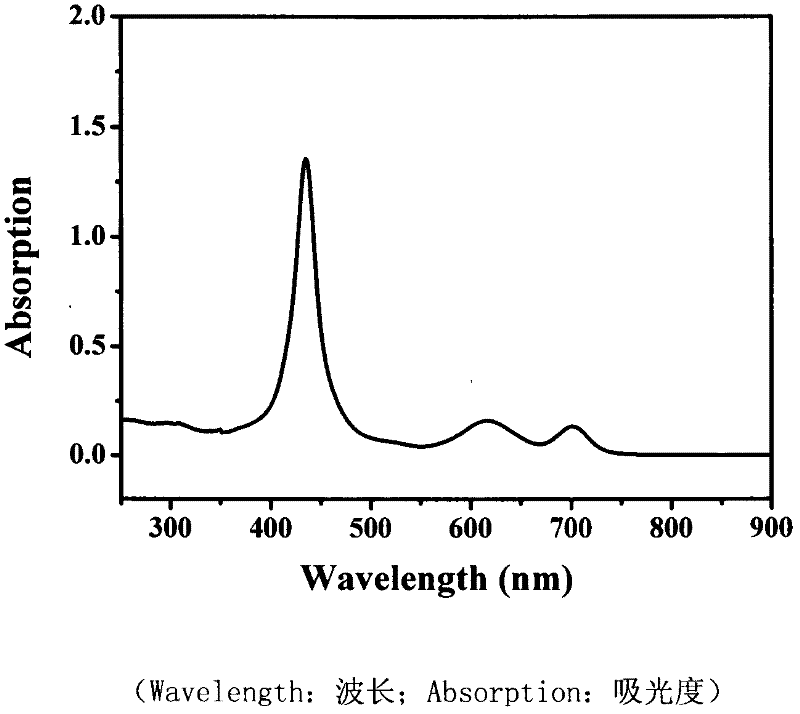
[0033] The nonlinear absorption and nonlinear refraction of 5,15-ferrocenyl-10,20-diphenylporphyrin were measured by Z-scan technique in pure chloroform with a concentration of 10 -4 mol / L -1 . The thickness of the cuvette containing the sample in the experiment is 1mm, and the beam waist radius at the focal point of the lens is w 0 is 40μm, the linear transmittance S of the small hole is 0.1, and the optical power density at the focal point of the lens is 7.593×10 9 W / cm 2. The entire system was calibrated with carbon disulfide prior to measurement. We under the same conditions for the pure solvent CHCl 3 Measurements were performed to confirm that the measured curves were all derived from the properties of the compounds and not influenced by the solvent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com