Complex catalyst system and application thereof to decomposition of naphthene hydrogen peroxide
A cycloalkyl hydroperoxide, catalyst technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, chemical/physical process, etc., can solve the problem of expensive, reactor wall fouling , catalyst deactivation and other problems to avoid precipitation and scaling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
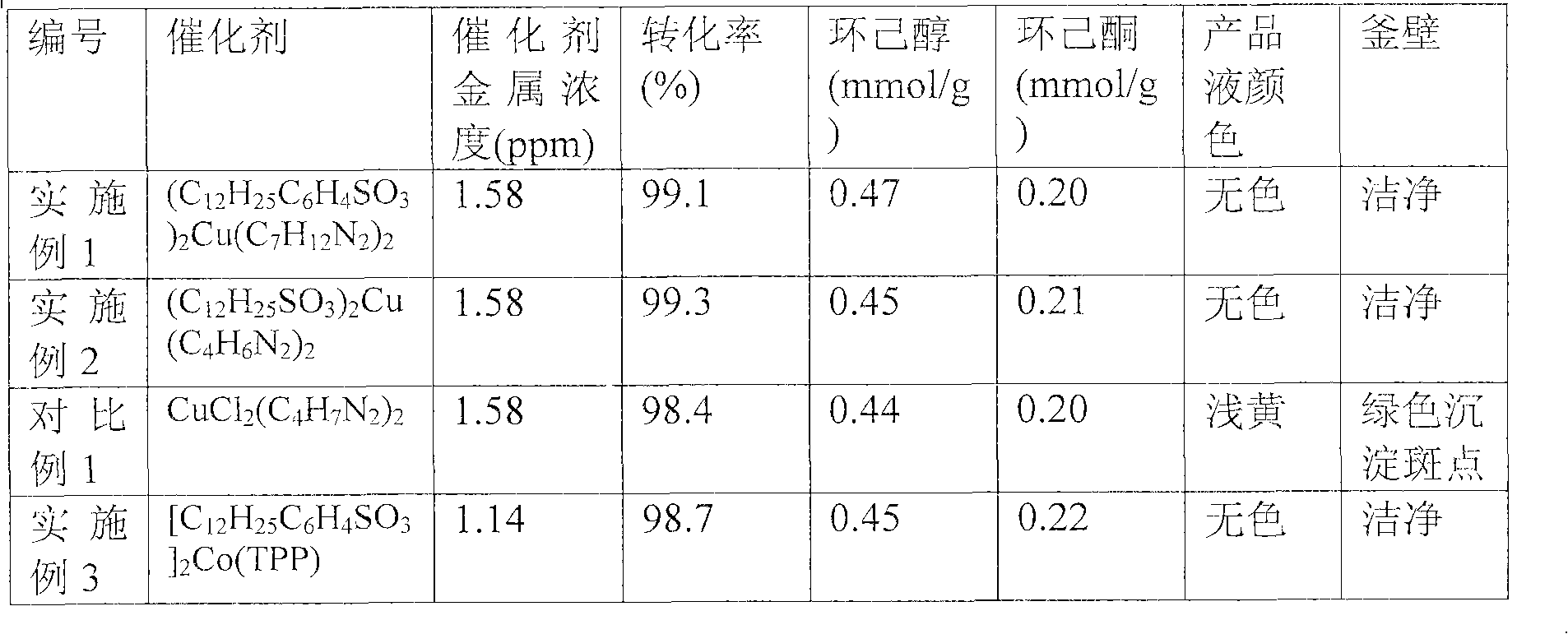
[0021] Reference Example 1: Preparation of Cyclohexane Oxidation Solution Containing Cyclohexyl Hydroperoxide
[0022] Cyclohexane oxidation was carried out in a 10-liter stainless steel reactor equipped with heating, mechanical stirring, ventilation and reflux. First add 7 liters of cyclohexane into the kettle, raise the temperature of the reaction kettle to 160°C under nitrogen, keep the pressure at 1.2MPa, change the nitrogen to air, the air flow rate is 8 liters / min, and stir the reaction at constant temperature for 1 hour. Analyze the cyclohexane oxidation solution prepared, this oxidation solution contains cyclohexyl hydroperoxide 0.44mmol / g, cyclohexanone 0.06mmol / g, cyclohexanol 0.12mmol / g, and oxidation by-products such as a small amount of organic acid and ester .
Embodiment 1
[0024] (1) Preparation of complex catalyst: N-butylimidazole complex copper dodecylbenzenesulfonate
[0025] Weigh 4.24g of dodecylbenzenesulfonic acid and 0.628g of copper hydroxide, add them to 20g of deionized water, heat and stir at 80°C for 20 minutes, then evaporate to dryness to obtain 4.62g of light white solid dodecylbenzene Copper sulfonate (C 12 h 25 C 6 h 4 SO 3 ) 2 Cu. Weigh 2.36g prepared copper dodecylbenzenesulfonate and 0.821g N-butylimidazole, add to reflux in 10ml ethanol for 30 minutes, evaporate to dryness, obtain 3.18g blue solid, this solid is through elemental analysis, Determine the composition as (C 12 h 25 C 6 h 4 SO 3 ) 2 Cu(C 7 h 12 N 2 ) 2 , Weigh 1.5g of this blue solid and dissolve it in 10ml of ethanol to make blue catalyst solution A.
[0026] (2) Catalytic decomposition of cyclohexyl hydroperoxide
[0027] The decomposition reaction is carried out in a 500ml stainless steel reaction kettle with a reflux water separation cond...
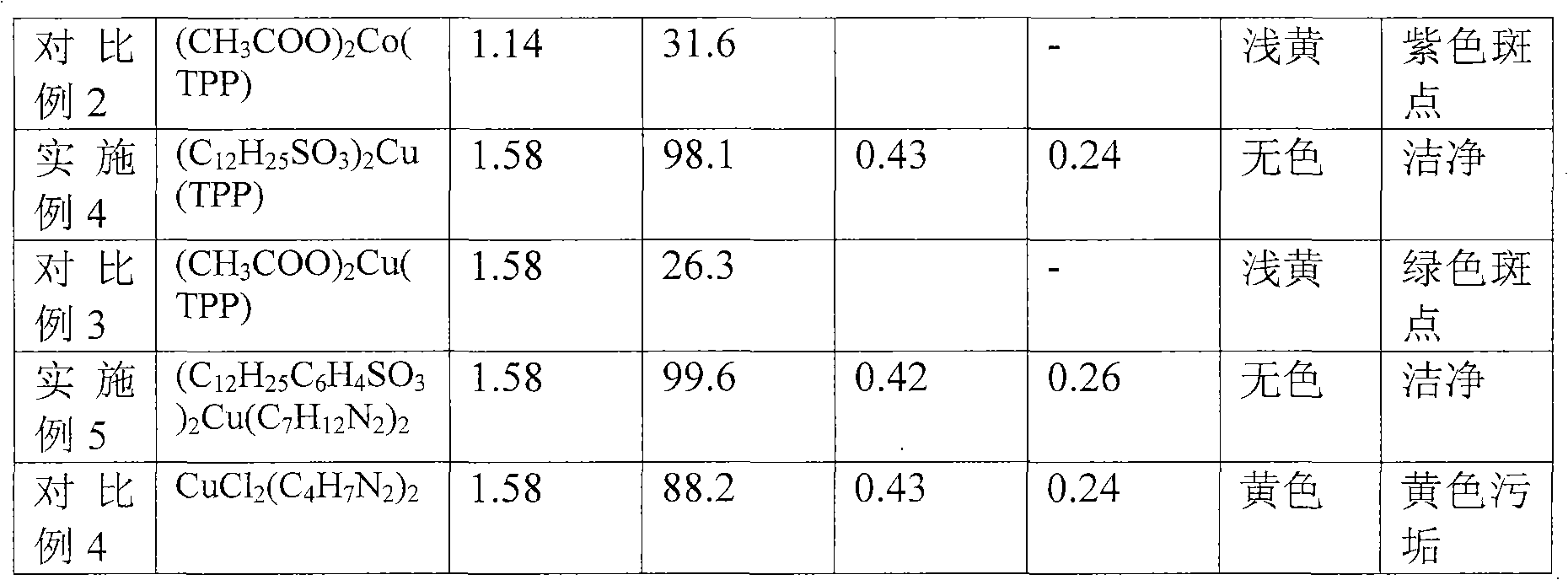
Embodiment 2
[0029] (1) Preparation of complex catalyst: N-methylimidazole complex copper dodecylsulfonate
[0030] Weigh 2.43g of sodium dodecylsulfonate and dissolve it in 49.8g of deionized water to obtain solution 1; then weigh 0.76g of copper chloride and dissolve it in 14.9g of deionized water to obtain solution 2. At room temperature under stirring conditions, solution 2 was slowly added dropwise to solution 1, and stirring was continued for 1 hour after the drop was completed, and a light blue solid precipitate was obtained. This precipitate is dried after centrifugation, deionized water washing 5 times, obtains 2.1g light blue powdery solid copper dodecylsulfonate (C 12 h 25 SO 3 ) 2 Cu.
[0031] Weigh 1.00g of the prepared copper dodecylsulfonate and 0.292g of N-methylimidazole, add it to 10ml of ethanol, heat and reflux at 80°C for 20 minutes, evaporate the solvent to obtain 1.28g of blue solid powder , this solid is determined to be composed of (C 12 h 25 SO 3 ) 2 Cu(C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com