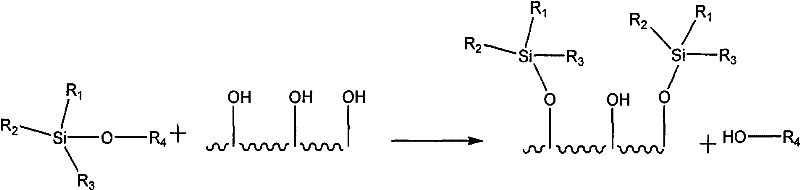
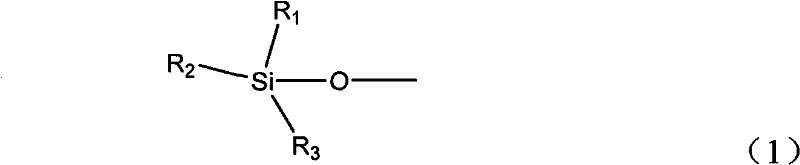
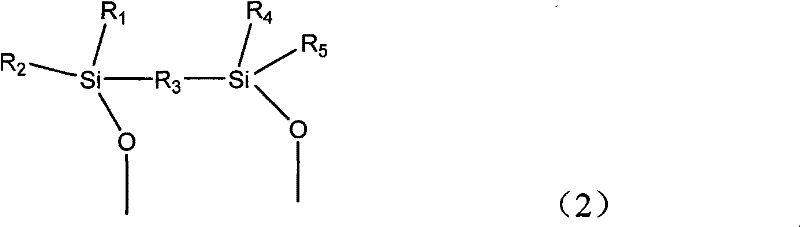
Regeneration method of copper, ruthenium, cobalt, nickel, palladium and platinum-based metal catalysts
A metal catalyst and catalyst technology, which is applied in the direction of catalyst regeneration/reactivation, metal/metal oxide/metal hydroxide catalyst, molecular sieve catalyst, etc., and can solve the problem of catalyst comprehensive performance degradation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] In this example, deactivated catalysts A-F were obtained; the composition and preparation method of each catalyst are listed in Table 1 in detail.
[0064] Table 1 Composition and preparation method of catalysts A to F
[0065]
[0066] The distribution of catalysts A to E is used in the hydrogenation of 3-hydroxypropionaldehyde to prepare 1,3-propanediol, the hydrogenation of ethanol to synthesize ethylamine, the gas-phase hydrogenation of dimethyl oxalate, the liquid-phase hydrogenation of benzene to prepare cyclohexane, and the cracking of C5 For the reaction of hydrodeykyne in the isoprene stream, the carbon distribution of the surface area of the catalyst after use is 9.2wt%, 6.5wt%, 11.2wt%, 4.5wt%, 18.2wt%.
Embodiment 2
[0068] A and B catalysts are used in a fixed bed reactor (diameter 15mm, length 400mm, with two temperature display control points). After deactivation, the temperature of both catalysts was raised to 120° C. in nitrogen, and then steam was passed through and kept for 2 hours. Subsequently, feed nitrogen containing 4vol% oxygen and heat up to 200°C, maintain for 2 hours, then switch to nitrogen containing 10vol% oxygen for 1h, then raise the temperature to 350°C, keep for 1h, switch to air, keep for 1h, and then heat up to 400°C, maintained for 2 hours, blowing nitrogen to blow down the temperature. After the reactor cools down to 110°C, hydrogen-containing methane is introduced, and the temperature is gradually raised to 300°C, while the hydrogen is gradually converted from 1 vol% to pure hydrogen. Then the temperature was lowered to 80°C, hydrogen gas containing 2vol% trimethylchlorosilane and 1vol% trimethylmethoxyalkane was passed into the reactor, the flow rate was contr...
Embodiment 3
[0070] Catalysts C, D and E are used in fixed-bed reactors, slurry-bed reactors and reaction-rectification reactors, and the catalysts are deactivated and then transferred from the reactors. Packed into a fixed bed reactor (diameter 35mm, length 1800mm, with six temperature display control points), and purged with argon after sealing. After purging in argon for 0.5h, the organic solvents of toluene and n-hexane in the liquid phase were introduced, and the washing was circulated for 3h, and the temperature was gradually changed from room temperature to 80°C. After the washing is stopped, different gases are passed into the catalyst: C, D and E catalysts are distributed as nitrogen containing oxygen, argon containing hydrogen, and methane hydrogen containing water vapor; the temperature is slowly raised from 80°C to 360°C, and then cooled . For catalyst C, nitrogen containing 30% hydrogen was passed through for reduction for 20 hours, and the temperature was raised from 40°C to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com