Extracellular yaluronidase from streptomyces koganeiensis
A hyaluronidase and hyaluronic acid technology, applied in the direction of enzymes, hydrolases, glycosylases, etc., can solve the problems of hyaluronidase difficulty, hyaluronidase instability, easy inactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
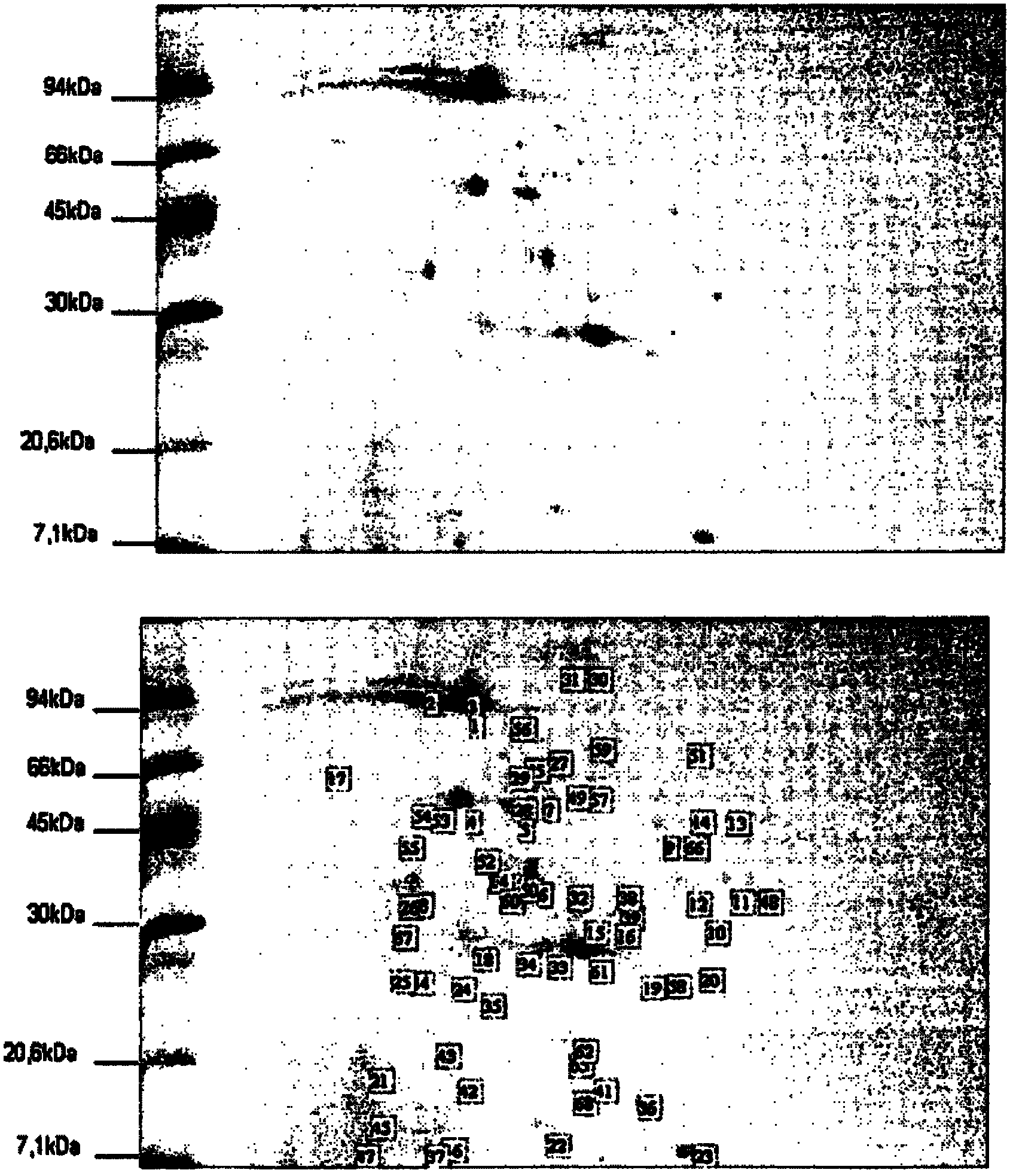
[0057] Embodiment 1 (reference example)—according to U.S. Patent US 4,258,134 to Streptomyces Preparation, Purification and Characterization of KOGANEIENSIS Hyaluronidase
[0058] Streptomyces S. koganeiensis (31394 ATCC) was fermented according to the method described in "Materials and Methods". After centrifugation to obtain the supernatant, filter with a suitable cross-flow filter device, and then perform DEAE cellulose weak anion exchange column chromatography. Briefly, 1.2kg of DEAE cellulose was equilibrated with 25mM sodium phosphate buffer, pH 7.0, and then loaded into the column, and then the fermentation supernatant was dialyzed with the same buffer, and then loaded with 25mM NaCl containing 250mM , pH7.0 phosphate buffer for elution. After the chromatography, the components containing hyaluronidase activity were collected and enriched by ultrafiltration. After enrichment, they were dialyzed with 10 times the volume of acetate buffer (pH 5.0), and then CM-cellul...
Embodiment 2
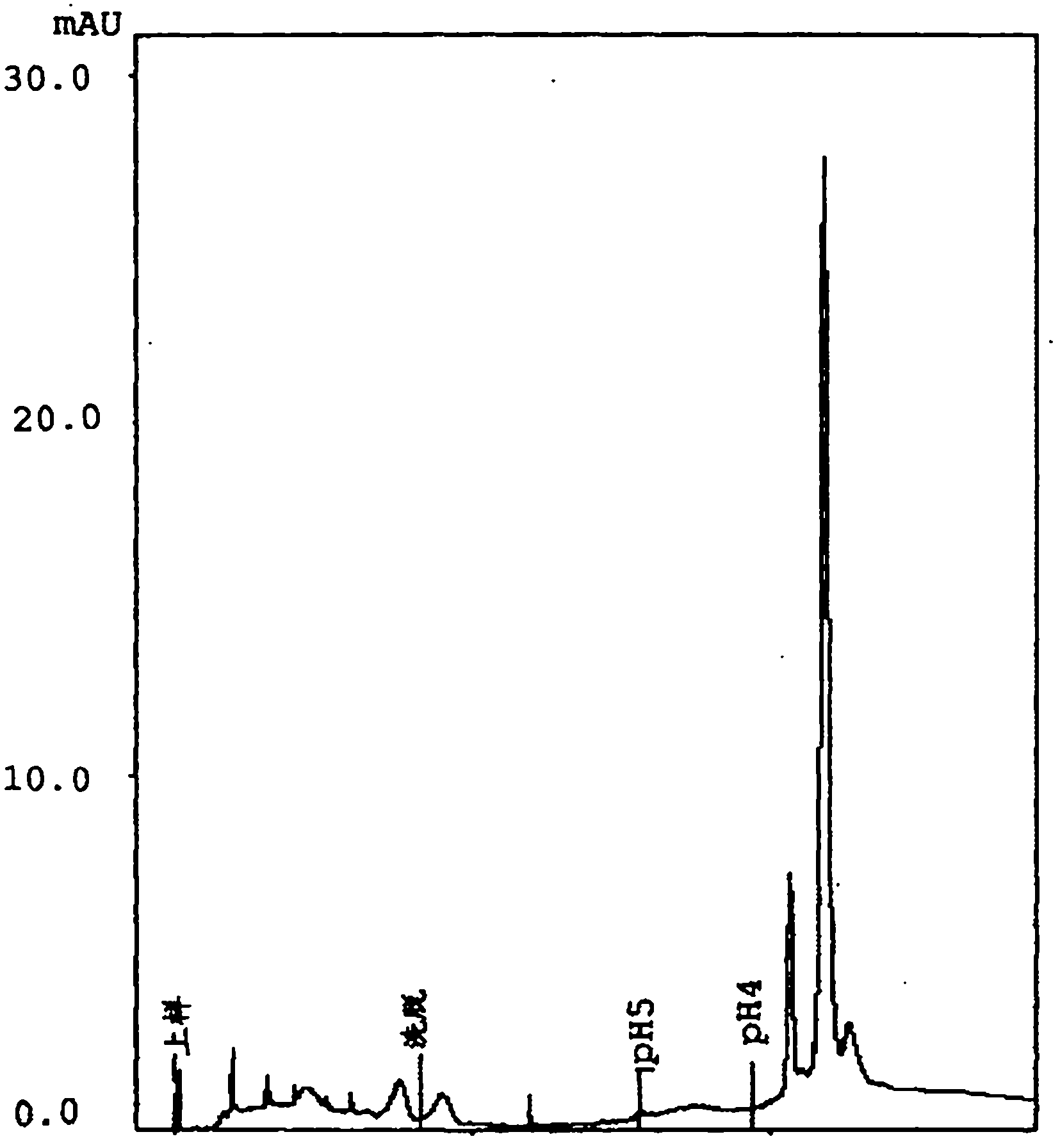
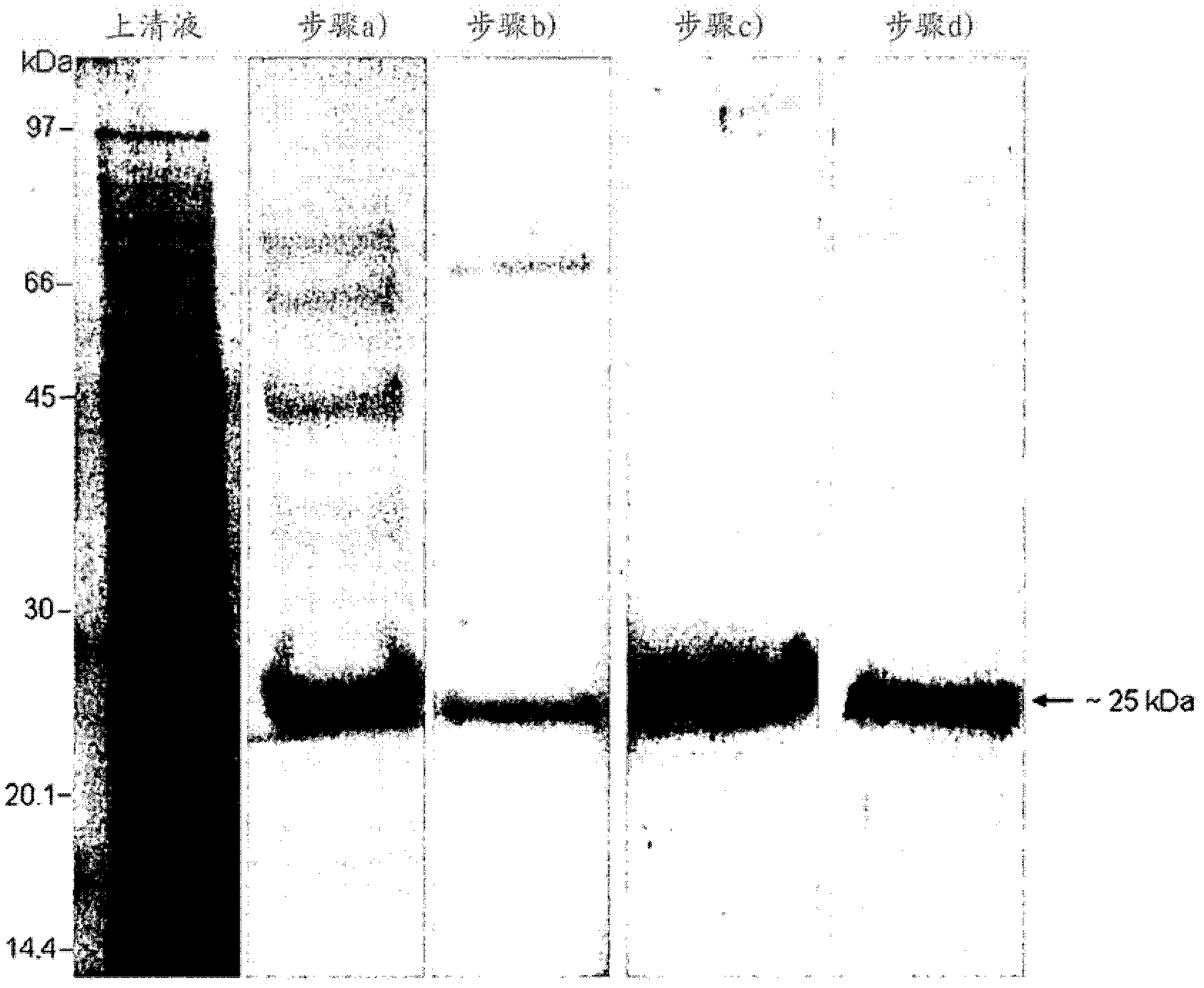
[0061] Embodiment 2-according to the present invention Streptomyces KOGANEIENSIS hyaluronidase is prepared, Purification and Characterization
[0062] 2A) Preparation and purification
[0063] Sample Preparation
[0064] Streptomyces koganeiensis (ATCC 31394) was fermented and cultured according to the method described in "Materials and Methods", and the resulting clear supernatant (about 30 L) was ultrafiltered with a polyethersulfone filter membrane with a molecular weight cut-off of 10-kDa, and concentrated After 10 times, the hyaluronidase activity was measured. The concentrated supernatant was dialyzed with 10 times volume of 50 mM sodium acetate solution, pH 4.0, and then used in step a).
[0065] Step a) weak cation exchange column chromatography
[0066] The concentrated and dialyzed supernatant was loaded on a 200ml CM-Sepharose An XK-50 chromatography column (GE Healthcare) with Fast Flow resin (GE Healthcare) was equilibrated with 10 bed volumes (BV) of 50 ...
Embodiment 3
[0095] Embodiment 3-preparation of pharmaceutical dosage form
[0096] Dosage Form 1 - Hydrophilic Gel
[0097]
[0098] Methylparaben and propylparaben were dissolved in pure water at 80°C. After cooling the solution to room temperature, add hyaluronidase, shake until completely dissolved, then add polyethylene glycol 400, continue shaking until dissolved. will carbomer Add 974P to the above solution, continue to shake until it is evenly dispersed and fully hydrated, then add TEA to obtain an aqueous phase gel. Finally, glycerin and propylene glycol are added with shaking.
[0099] Dosage Form 2-Hydrophilic Cream (O / W Emulsion)
[0100] components
[0101] For the preparation of the oil phase, liquid paraffin, stearic acid and Tefose 1500 were shaken and melted at 50°C. In addition, for the preparation of the aqueous phase, an initial solution of methyl p-hydroxybenzoate at 80°C was used, then cooled to room temperature, glycerin and hyaluronidase were ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com