Targeting polymer micelle containing acid-sensitive subsurface, and preparation method thereof
A polymer glue and polymer technology, applied in the fields of pharmacy, biomedical engineering, and chemistry, can solve the problems that fluorescent organic dye molecules are prone to photobleaching and cannot effectively overcome the masking, and achieve the effect of real-time monitoring of fluorescence imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]Synthesis of Folic Acid Modified Triblock Copolymer FA-PEG-P(Asp-dip)-CA with Acid Sensitive Middle Block
[0028] (1) Synthesis of polyethylene glycol (Allyl-PEG-OH) with an allyl group at one end and a hydroxyl group at the other end
[0029] A solution of potassium naphthyl (4 mL) in tetrahydrofuran and allyl alcohol (0.75 mL) was mixed evenly and stirred in a dry reaction flask for 15 minutes. Then under the protection of argon, anhydrous tetrahydrofuran (20 mL) and 18-crown-6 tetrahydrofuran solution (containing 1.5 g 18-crown-6 and 5 mL anhydrous tetrahydrofuran) were added, stirred for 15 minutes, and the mixture was placed in Cool in an ice-salt bath, slowly pass through dry ethylene oxide, keep the low temperature for 24 hours to keep the polymerization reaction going, and continue the reaction at room temperature for 72 hours.
[0030] (2) Polyethylene glycol with an allyl group at one end and an amino group at the other end (Allyl-PEG-NH 2 )Synthesis
[003...
Embodiment 2
[0046] Preparation of targeted polymeric micelles with acid-sensitive subsurface
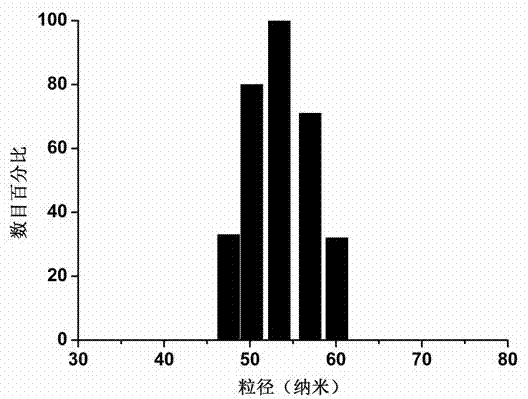
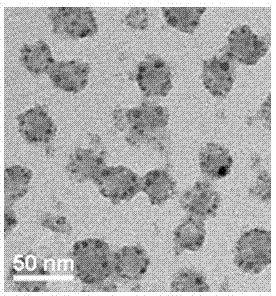
[0047] (1) Sample preparation of loaded QD micelles: a mixture of FA-PEG-P(Asp-dip)-CA (2.0 mg) and PEG-P(Asp-dip)-CA (8.0 mg) was dissolved in tetrahydrofuran (THF, 1.0 mL), added dropwise into PBS (10 mL, pH=5.0) under ultrasonic conditions, stirred overnight, THF was naturally volatilized, filtered with a filter membrane with a pore size of 0.22 μm, and then added negative electron dots CdSe / ZnS (100 μL, 8 μmoL / L), mix well and rest for 30 minutes, adjust the pH to 7.4, and centrifuge at 6000r / min to remove unloaded quantum dots.
[0048] (2) Sample preparation of loaded QDs and paclitaxel micelles: a mixture of FA-PEG-P(Asp-dip)-CA (2.0 mg) and PEG-P(Asp-dip)-CA (8.0 mg) was mixed with PTX (1.0 mg) was dissolved in tetrahydrofuran (THF, 1.0 mL), added dropwise into PBS (10 mL, pH=5.0) under ultrasonication, stirred overnight, THF was naturally volatilized, and PTX was filtered through a fil...
Embodiment 4
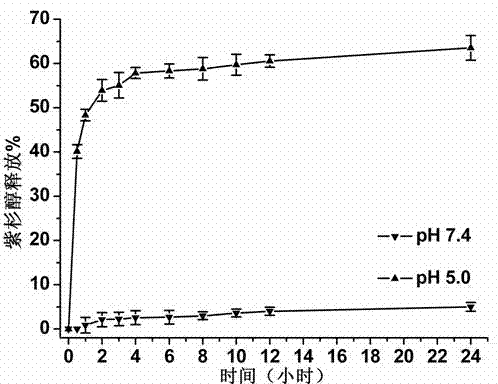
[0053] In vitro drug release test of targeted polymeric micelles with acid-sensitive subsurface (take paclitaxel-loaded micelles as an example)
[0054] The in vitro release experiment of paclitaxel was done by dialysis. The newly prepared paclitaxel-loaded PEG-P(Asp-dip)-CA micelles were divided into two parts, one part maintained its pH value at 5.0, and the other part was adjusted to 7.4. The samples at each pH value were divided into three (parallel experiments) and loaded into dialysis bags with a molecular weight cut-off of 14,000 Da, and the dialysis bags were placed in 100 mL of PBS buffer with the same pH value. Placed in a shaker at 37°C at a speed of 75 r / min, samples were taken at set time points, and then the same volume of fresh buffer solution was added. The concentration of the sample was detected by HPLC, and the cumulative release at different time points was calculated. The chromatographic column model used in HPLC is Ultimate from Welch Materials Company ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com