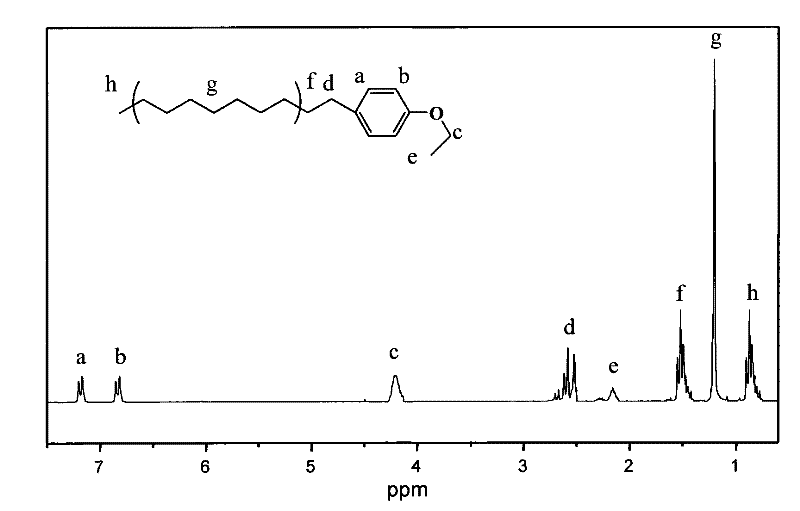
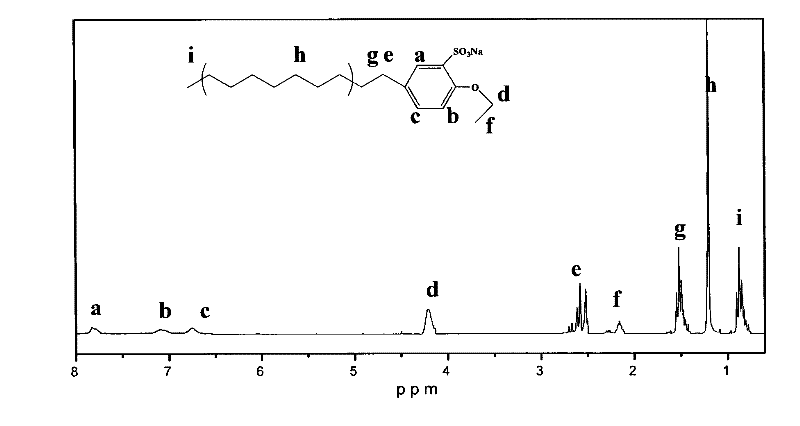
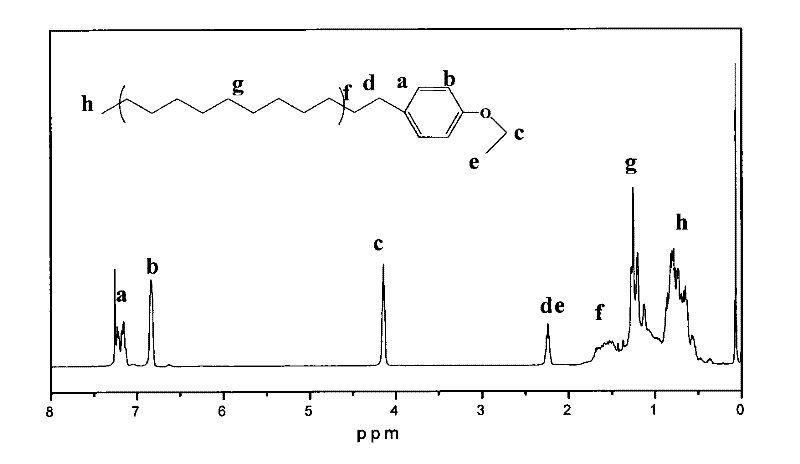
Alkylbenzene sulfonate Gemini surfactant and preparation method thereof
A technology of alkylbenzene sulfonate and surfactant, applied in the field of alkylbenzene sulfonate Gemini surfactant and its preparation, can solve the problems of complex synthesis process, low critical micelle concentration surface tension, and high cost, To achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1 (10-3-10 synthesis)
[0060] Step 1: 2.82g (30mmol) phenol, 2.02g (10mmol) 1,3-dibromopropane, 1.2g (30mmol) sodium hydroxide and 0.16g (0.5mmol) tetrabutylammonium bromide are dissolved in 25ml water, and Raise the temperature to 95°C, keep a slight boil and stir vigorously. The mixture was refluxed for three hours and then cooled, extracted with anhydrous diethyl ether (20ml×3) at room temperature, combined and extracted organic phases, and dried over anhydrous sodium sulfate for 12 hours, the organic solvent was distilled off under reduced pressure, and the residue was dissolved in anhydrous Crystallized in water and ethanol, 1.76 g of product was isolated with a yield of 77%. The product is white needle-like crystals.
[0061] Step 2: 6.15g (27mmol) diethers were added dropwise to 11.88g (54mmol) of brominated n-decane and 7.12g (54mmol) of aluminum trichloride in anhydrous 1,2-dichloroethane solution, at 0.3 Stir the reaction at MPa and 80°C for 5 h...
Embodiment 2
[0063] Embodiment 2 (12-3-12 synthesis)
[0064] Step 1: 2.82g (30mmol) phenol, 2.02g (10mmol) 1,3-dibromopropane, 1.2g (30mmol) sodium hydroxide and 0.16g (0.5mmol) tetrabutylammonium bromide are dissolved in 25ml water, and Raise the temperature to 95°C, keep a slight boil and stir vigorously. The mixture was refluxed for three hours and then cooled, extracted with anhydrous diethyl ether (20ml×3) at room temperature, combined and extracted organic phases, and dried over anhydrous sodium sulfate for 12 hours, the organic solvent was distilled off under reduced pressure, and the residue was dissolved in anhydrous Crystallized in water and ethanol, 1.76 g of product was isolated with a yield of 77%. The product is white needle-like crystals.
[0065] Step 2: In, add 6.13g (27mmol) bis-ether dropwise to 13.40g (54mmol) of n-dodecane bromide and 7.12g (54mmol) of aluminum trichloride in anhydrous 1,2-dichloroethane solution , the reaction was stirred at 0.3MPa and 80°C for 5 ...
experiment example 1
[0068] Surface tension curves were measured by the drop volume method. To achieve surface adsorption equilibrium, the formation of each droplet is divided into two steps: first, approximately 90% of the entire droplet volume is quickly extruded, and then the droplet hangs for a sufficient time until the entire droplet falls down automatically. Each surface tension value was averaged from at least three experimental values. The measurement temperature is 25.0±0.1°C. Triple distilled water was used for the solution configuration in this experiment.
[0069] Example 2
[0070] In this experiment, the interfacial tension of the hexadecane / water interface was measured by the spinning drop method (TEX500 rotating interfacial tensiometer), the sample rotation speed was maintained at 5000 rpm, and the measurement temperature was 30.0±0.1°C. Triple distilled water was used for the solution configuration in this experiment. The interfacial tension value can be calculated by the foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com