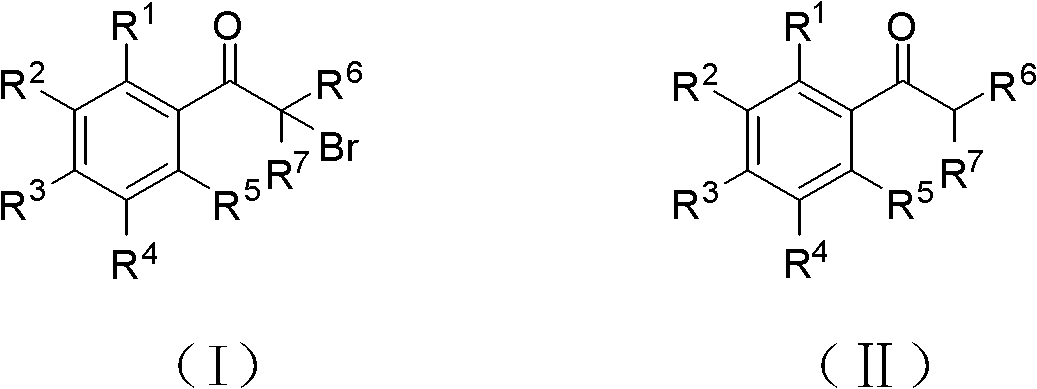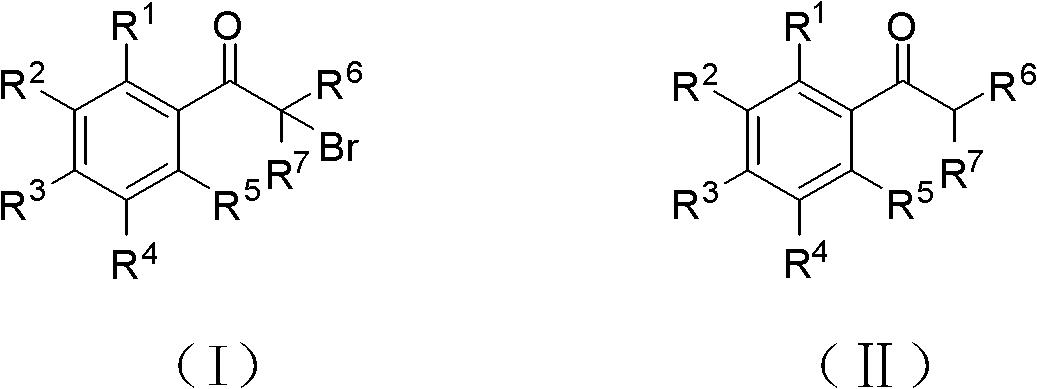Method for synthesizing alpha-brominated aromatic ketones compound
A synthesis method and technology of aromatic ketones, which are applied in the field of synthesis of aromatic ketones, can solve the problems of unsafe operation, increased production cost, and high cost, and achieve the effects of high economic benefit, convenient operation, and easy acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 12g (0.1mol) acetophenone, 1.21g (5mmol) copper nitrate trihydrate, 14mL (hydrogen bromide 0.112mol) 8.0mol / L hydrobromic acid aqueous solution into an autoclave lined with polytetrafluoroethylene, and feed 1:1 air and oxygen mixture to a pressure of 7-10atm, stir rapidly, react at 70°C for 2 hours, then stop the reaction, after cooling, a light yellow solid precipitates, filter the reaction solution, wash the filter cake with water until neutral, and dry , 1.89 g of the product α-bromoacetophenone was obtained, and the yield was 95%.
Embodiment 2
[0031] Add 1.38g (10mmol) p-fluoroacetophenone, 0.121g (0.5mmol) copper nitrate trihydrate into the flask, and feed air under normal pressure, and slowly add 1.4mL (hydrogen bromide 11.2mmol ) 8.0mol / L hydrobromic acid aqueous solution, add dropwise completely in about one and a half hours, continue to react for about 10 hours after dropping, carry out TLC follow-up detection, after the reaction is complete, the reaction solution is separated through silica gel column chromatography, with volume ratio 3: 1 Petroleum ether (60-90° C.):dichloromethane mixture was used as eluent to obtain 0.46 g of the product α-bromo-p-fluoroacetophenone with a yield of 21%.
Embodiment 3
[0033] Add 1.56g (10mmol) 2,4-difluoroacetophenone and 0.121g (0.5mmol) copper nitrate trihydrate to the flask, feed oxygen, control the temperature at 95°C under normal pressure, and slowly add 1.4mL to it (hydrogen bromide 11.2mmol) 8.0mol / L hydrobromic acid aqueous solution, add dropwise completely in about one and a half hours. Using a volume ratio of 3:1 petroleum ether (60-90°C):dichloromethane mixture as eluent, separated by silica gel column chromatography to obtain 1.90 g of the product α-bromo 2,4-difluoroacetophenone, Yield 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com