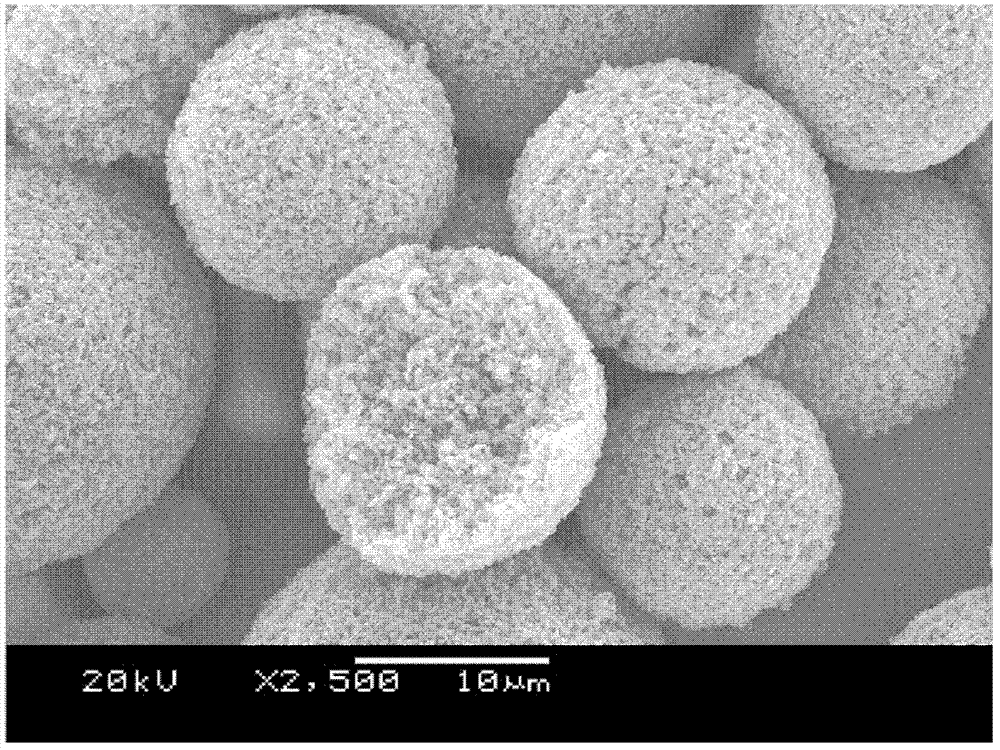
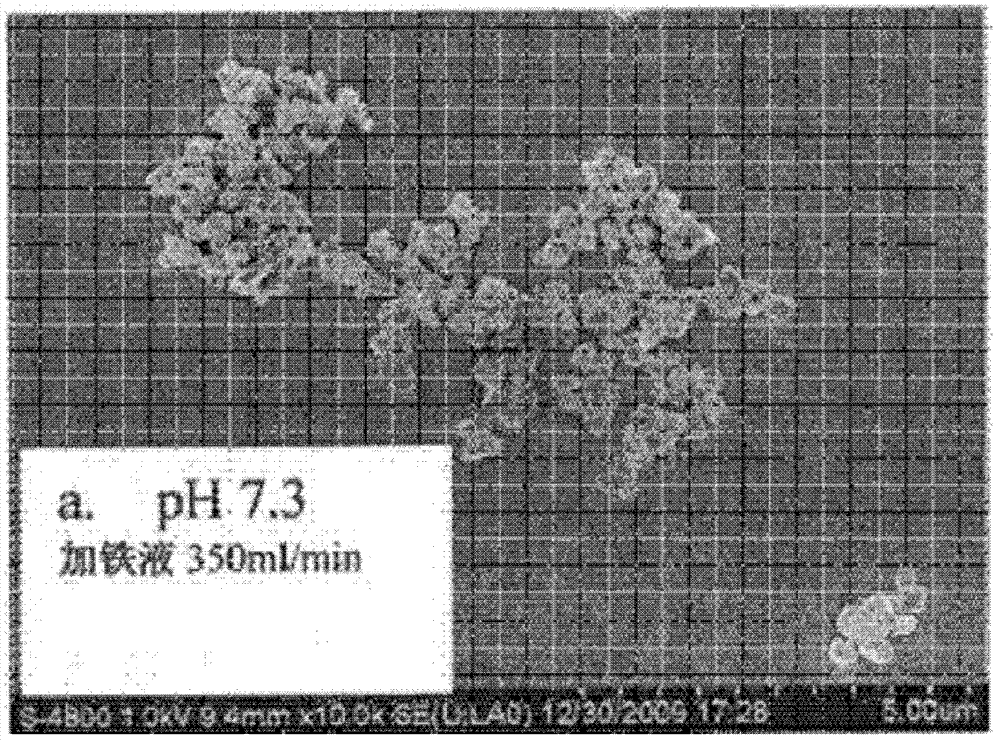
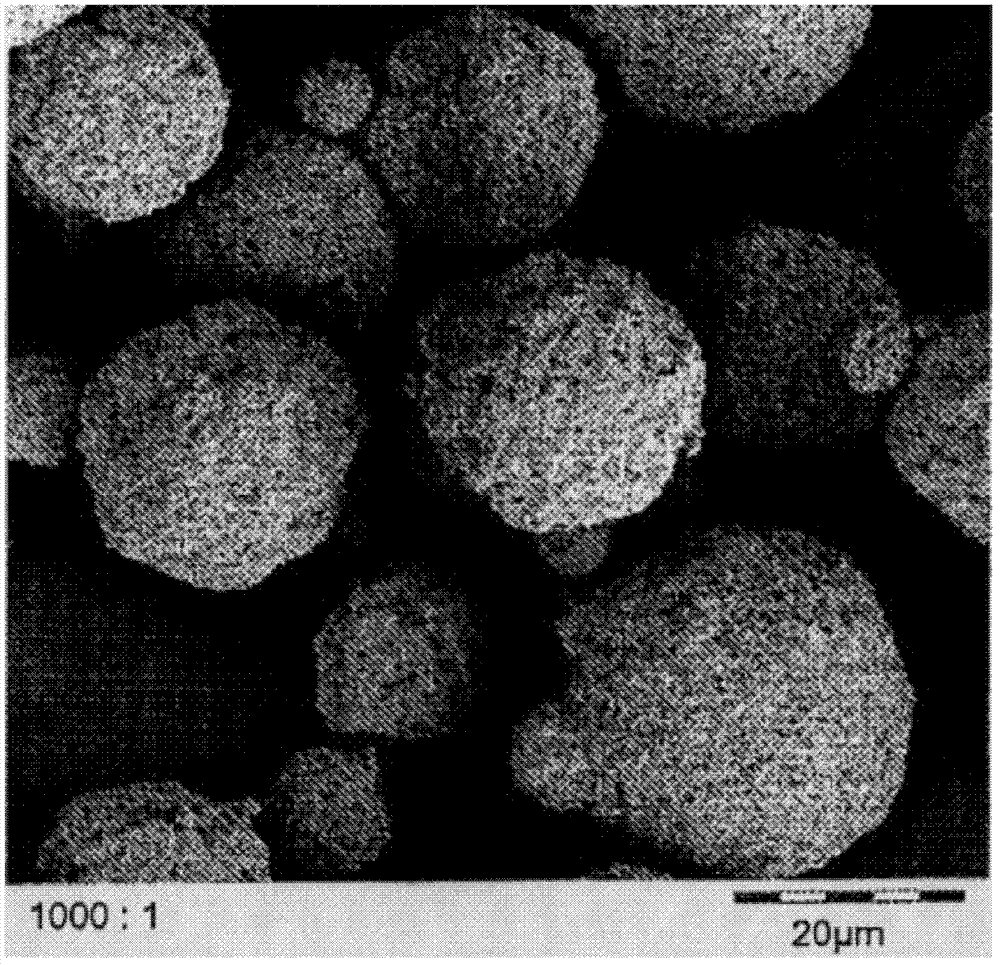
LiFePO4 (lithium iron phosphate) positive electrode material with specific morphology and structure and lithium secondary battery
A technology of lithium ferrous phosphate and carbon-coated lithium ferrous phosphate, which is used in battery electrodes, structural parts, nanotechnology for materials and surface science, etc. , Affect the dispersion uniformity of the carbon coating layer, coating thickness and other issues, to achieve excellent capacity retention, ensure electrical conductivity, and improve the effect of high current charge and discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The technical solution for solving the above-mentioned problems is: the preparation method of carbon-coated lithium iron phosphate with a specific microscopic morphology structure passes through the following steps:
[0072] (1) Preparation of flaky particles of lithium iron phosphate:
[0073] Lithium salt solution, ferrous salt solution and phosphorus source solution are heated to 120°C-180°C under stirring conditions at a rate of 20-200°C / hour (preferably 50-80°C / hour, more preferably 60°C) °C / hour), heat preservation for 2-15 hours (preferably 4-12 hours, more preferably 6 hours), filter after cooling, and wash the filter cake.
[0074] (2) Preparation of sugar-containing spherical or spherical-like lithium iron phosphate:
[0075] Add the sugar raw material aqueous solution to the lithium iron phosphate of the flaky particles prepared in step (1) until the solid content of the mixed liquid is 10-50%, preferably 10%, stir uniformly, and spray dry to obtain;
[0076] Wherein,...
Embodiment 1
[0168] The preparation of carbon-coated lithium iron phosphate includes the following steps:
[0169] (1) Configure a lithium salt solution with a lithium content of 25.34g / L;
[0170] (2) Weigh 5531.0 grams of 62.0% ferrous chloride and prepare 36 liters of ferrous salt solution (Fe 2+ Concentration: 58.3g / L);
[0171] (3) Weigh 14071.6 grams of 98.0% ammonium phosphate trihydrate to prepare 5 liters of phosphorus source solution (PO 4 3- Concentration: 685.9g / L);
[0172] (4) Take 3 liters of lithium salt solution, 3.5 liters of ferrous salt solution and 0.5 liters of phosphorus source solution, add them to the reactor within 10 minutes under stirring, continue stirring, and heat up to 150 at a rate of 200°C / hour ℃, heat preservation for 12 hours, cool at a cooling rate of 60℃ / hour, release and filter, take the filter cake, and obtain lithium iron phosphate primary particles;
[0173] (5) Wash the filter cake twice, until no lithium ions are detected in the filter cake washing liquid...
Embodiment 2
[0183] (1) Prepare a lithium content of 26.95g / L to obtain a lithium salt solution;
[0184] (2) Weigh 3568.6 grams of 98.3% ferrous sulfate to prepare 35 liters of ferrous solution (Fe 2+ The concentration is 54.8g / L);
[0185] (3) Weigh 4825.3 grams of 85.3% phosphoric acid to prepare 5 liters of phosphorus solution (PO 4 3- The concentration is 798.0g / L);
[0186] (4) Add 3 liters of lithium salt solution, 3.5 liters of ferrous solution and 0.5 liters of phosphorus solution to the reaction kettle within 3 minutes under stirring, continue stirring, and heat to 220°C at a rate of 20°C / hour. Keep the temperature for 4 hours, cool at a cooling rate of 300°C / hour, and then release and filter to obtain a filter cake, namely lithium iron phosphate primary particles;
[0187] (5) Wash and filter the filter cake 5 times until no lithium ions are detected in the washing filtrate, take the washed filter cake; dissolve 110 grams of glucose (20%) in 5 liters of water, put in the filter cake, mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com