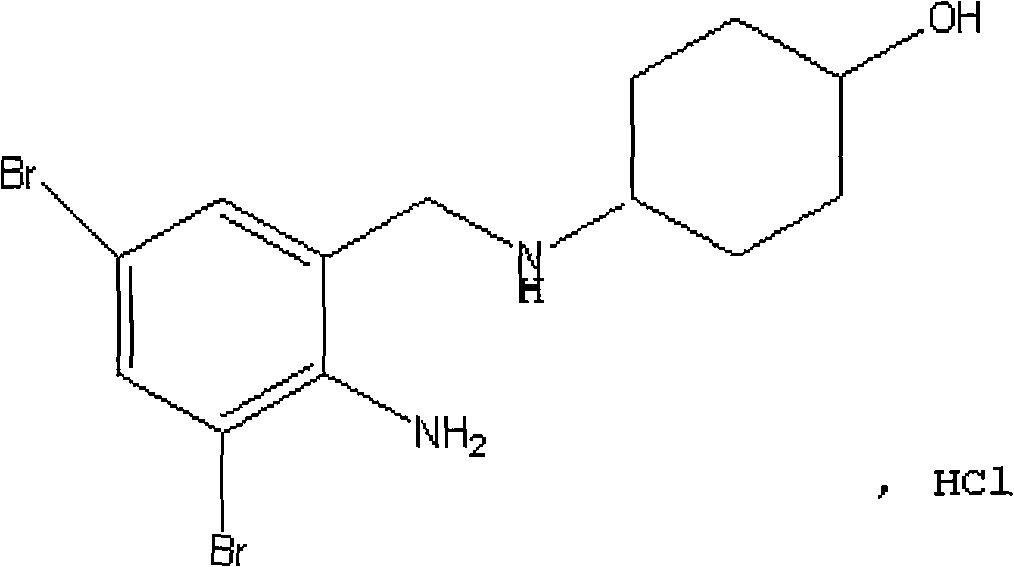
Refining method of hydrochloric acid ambroxol compound
A kind of technology of ambroxol hydrochloride and crude product of ambroxol hydrochloride, which is applied in the field of ambroxol hydrochloride compound and its preparation method, can solve the problems such as the content reduction of the active ingredient of the drug, the purity of the drug does not meet the requirements, and the improper control of the production process. Improving respiratory conditions, simple and easy refining process, and improving the effect of clinical adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The refining of embodiment 1 ambroxol hydrochloride
[0049] The ambroxol hydrochloride crude product (its HPLC purity is 92.52%) that 10g is made according to prior art method is dissolved in 80ml methanol, stirs, makes it dissolve completely, then adds the gac of 0.3g, stirs and adsorbs for 20 minutes, filters and removes charcoal, collect the filtrate; add 10g aluminum oxide after concentration and stir evenly, add to the upper end of the preparative chromatographic column after the solvent is evaporated, and then use the preparative chromatographic column to separate and purify the filtrate, wherein the mobile phase used in the chromatographic column is a volume ratio of 1:1.5 mixed solvent of acetonitrile and water, the flow rate is 0.2ml / min, the stationary phase filler is ICN allumina N neutral alumina with a particle diameter of 18-32 μm and a pore diameter of 6nm, the column temperature is room temperature, and the wavelength is 248nm. Deliquified, concentrat...
Embodiment 2
[0059] The refining of embodiment 2 ambroxol hydrochloride
[0060] 10g ambroxol hydrochloride crude drug (pharmaceutical factory: Shandong Luoxin Pharmaceutical Co., Ltd., lot number: 20091102; HPLC purity is 92.76%) is dissolved in 100ml ethanol under heating, stirs, and it is dissolved completely, then adds 0.2 g of activated carbon, stirred and adsorbed for 15 minutes, filtered for decarbonization, and collected the filtrate; after concentrating, add 10 g of alumina and stir evenly, evaporate the solvent and add it to the upper end of the preparative chromatographic column, and then use the preparative chromatographic column to separate and purify the filtrate. Wherein the mobile phase used in the chromatographic column is a mixed solvent of methanol and water with a volume ratio of 1:1.2, the flow rate is 2ml / min, and the stationary phase filler is a Baker column chromatography special neutral alumina with a particle size of 50-200 μm and a pore size of 6 nm. The column...
Embodiment 3
[0065] The refining of embodiment 3 ambroxol hydrochloride
[0066] 10g ambroxol hydrochloride bulk drug (pharmaceutical factory: Shandong Luoxin Pharmaceutical Co., Ltd., batch number: 20091102; HPLC purity is 92.76%) is dissolved in 120ml ethylene glycol under heating, stirs, and it is dissolved completely, then Add 0.4g of activated carbon, stir and adsorb for 15 minutes, filter and decarbonize, and collect the filtrate; after concentration, add 15g of alumina and stir evenly, evaporate the solvent and add to the upper end of the preparative chromatographic column, and then separate and purify the filtrate. The mobile phase used is a mixed solvent of methanol and water with a volume ratio of 1:1.8, the flow rate is 1ml / min, the stationary phase filler is ICN allumina N neutral alumina with a particle size of 18-32 μm and a pore size of 6 nm, and the column temperature is Room temperature, wavelength 248nm, collect the eluate, and concentrate under reduced pressure at 50°C. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com