Baicalin metal complex and preparation method and application thereof
A technology of baicalin metal and metal complexes, which is applied in the preparation of sugar derivatives, iron group organic compounds without C-metal bonds, and 1/11 group organic compounds without C-metal bonds, etc., which can solve the structure of baicalin Easy to be destroyed, high cost of large-scale production, large environmental pollution and other problems, to achieve the effect of avoiding the possibility of metal hydroxides, good operator and environmental safety, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
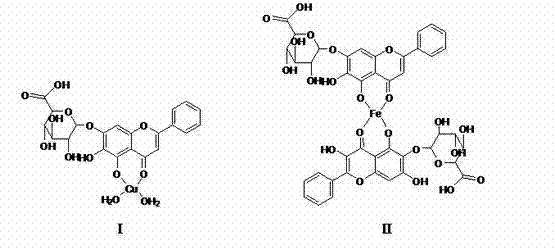
[0017] Embodiment 1, the preparation of baicalin metal complex
[0018] (1) Preparation of baicalin copper complex
[0019] Add 1% sodium bicarbonate aqueous solution to baicalin, the molar ratio of baicalin to sodium bicarbonate is 1:1, stir to completely dissolve baicalin, and then follow the baicalin and Cu(NO 3 ) 2 The molar ratio is 1:2 by adding Cu(NO 3 ) 2 , stirred and reacted for 4 hours at a temperature of 20° C., filtered, and the resulting brownish-yellow precipitate was washed with water and dried to obtain the baicalin copper complex with a yield of 73.93%.
[0020] (2) Preparation of baicalin aluminum complex
[0021] Add the sodium acetate aqueous solution that mass percent concentration is 1% to baicalin, the mol ratio of baicalin and sodium acetate is 1:1, stir to make baicalin dissolve completely, then according to baicalin and Al 2 (SO 4 ) 3 The molar ratio is 2:1 by adding Al 2 (SO 4 ) 3 , stirred and reacted at 40°C for 6 hours, filtered, and t...
Embodiment 2
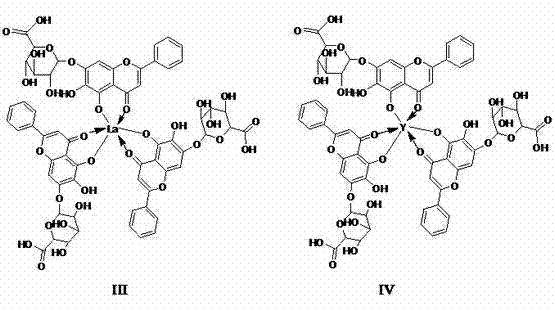
[0029] Embodiment 2, the structural characterization of baicalin metal complexes
[0030] (1) UV characterization results
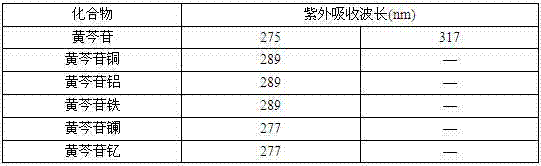
[0031] The five baicalin metal complexes prepared in Example 1 were respectively dissolved in 1% NaOH aqueous solution by mass percentage, and the maximum absorption wavelength was measured with an ultraviolet spectrophotometer. The results are shown in Table 1.
[0032] Table 1 UV absorption wavelengths of baicalin and its metal complexes
[0033]
[0034] It can be seen from Table 1 that baicalin has two absorption peaks at 275nm and 317nm respectively, while copper baicalin, aluminum baicalin, iron baicalin, lanthanum baicalin and yttrium baicalin complexes have only one absorption peak at 289nm or 277nm , that is, the absorption peak at 317nm of baicalin disappeared, and the original absorption peak at 275nm was red-shifted. After baicalin forms a complex, the degree of delocalization of electrons in the molecule increases and metal ions have a...
Embodiment 3
[0059] Embodiment 3, the acute toxicity of baicalin metal complex
[0060] Dissolve the five baicalin metal complexes prepared in Example 1 with 1% NaOH aqueous solution to make a 10% solution, and gavage each mouse according to the maximum tolerated dose method Give 5g / kg, observe continuously for two weeks, and record the activity and death of mice.
[0061] The results show that after intragastric administration, all mouse activities weakened slightly, but recovered in a short time; all mice grew well within two weeks, no toxic reaction was observed, and no mice died; illustration embodiment 1 LD of the prepared five kinds of baicalin metal complexes in mice 50 All greater than 5g / kg. According to acute toxicity (LD 50 ) dosage grading, the oral administration of the above five complexes belongs to the actual non-toxic range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com