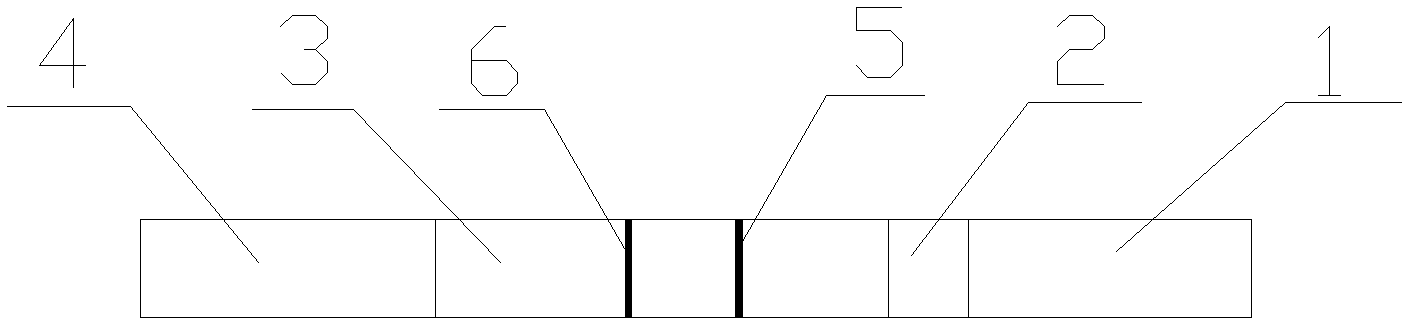
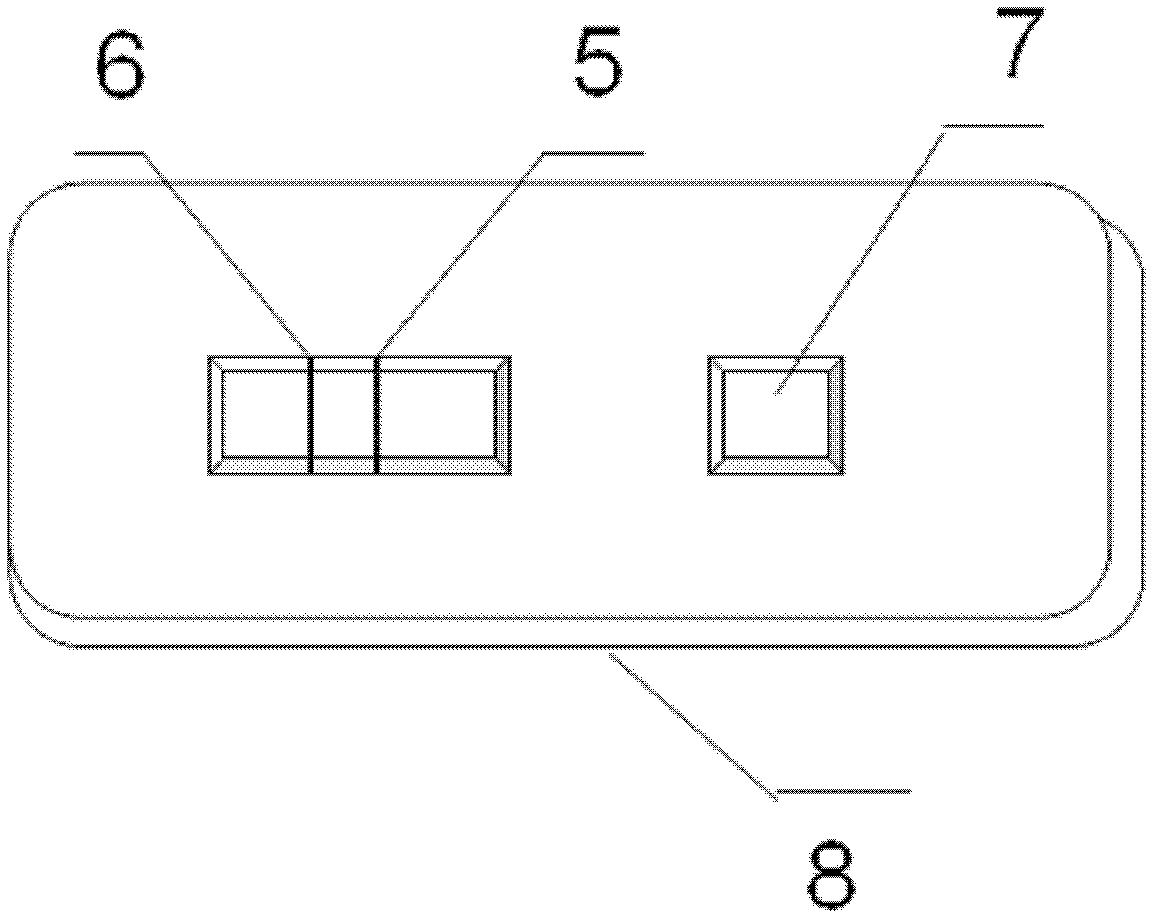
Microscale urinary albumin colloidal gold detection kit and preparation technology thereof
A technology for detecting kits and urinary albumin, which is applied in biological testing, measuring devices, material inspection products, etc., and can solve the problems of unfavorable clinician diagnosis and curative effect observation, high cost, and long duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 trace urine albumin colloidal gold detection kit
[0068] 1. Reagent source:
[0069] Anti-human albumin monoclonal antibody I, anti-human albumin monoclonal antibody II, goat anti-mouse IgG polyclonal antibody (purchased from MAXMED LABORATORIES INC., USA)
[0070] Nitrocellulose film, glass fiber paper (purchased from SARTORIUS, Germany Sartorius Company)
[0071] 2. Preparation steps:
[0072] method 1:
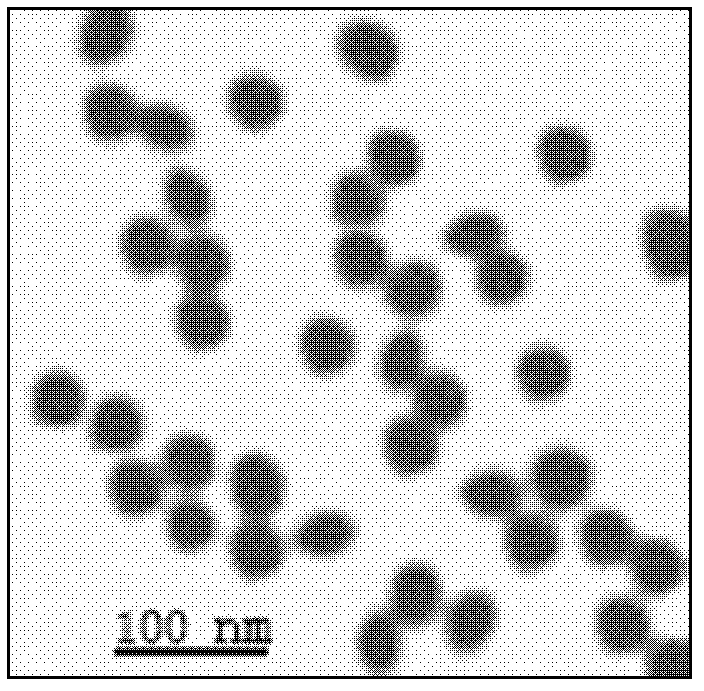
[0073] 1. Preparation of colloidal gold:
[0074] a) Reduction of chloroauric acid solution for the first time: HAuCl of 6ml 0.0164mol / L 4 The aqueous solution was added to 200ml of double-distilled water, heated and boiled for 30 minutes, then stirred slowly and accurately added 50ml of 0.016mol / L trisodium citrate aqueous solution. Cool to room temperature after ultrasonic oscillation for 2 minutes, the frequency of ultrasonic oscillation is 25KHZ, and a colloidal gold original solution with a particle size of about 15nm is pre...
Embodiment 2
[0121] The sensitivity experiment of embodiment 2 trace urine albumin colloidal gold detection kit
[0122] Detection method:
[0123] 1. Take the microalbuminuria colloidal gold detection kit prepared in Example 1, and place the kit on a horizontal platform.
[0124] 2. Take the urine sample to be tested with a sample pipette, and then drop 2-3 drops (about 100 μl) of the sample into the sample hole of the kit, and use a different pipette for each different sample.
[0125] 3. Observation results: Interpret the results 10-15 minutes after adding the sample.
[0126] Test sample:
[0127] Quality control reference substances with human albumin concentrations of 7.5 μg / ml, 10 μg / ml, 15 μg / ml, and 30 μg / ml were prepared as standards.
[0128] Test results:
[0129] Table 1 Kit sensitivity results
[0130]
[0131] The results in Table 1 confirm that the microalbuminuria colloidal gold detection kit prepared by the present invention has a sensitivity of 15 μg / ml, which me...
Embodiment 3
[0132] The specificity experiment of embodiment 3 trace urine albumin colloidal gold detection kit
[0133] Get the detection kit prepared in Example 1, add samples respectively according to the following table and detect, and the detection results are shown in Table 2:
[0134] Table 2 The specificity test results of microalbuminuria colloidal gold detection kit
[0135]
[0136] It can be seen from the above table that the microalbuminuria colloidal gold detection kit of the present invention has no cross-reaction to 500 μg / ml hemoglobin, 20 μg / ml acetaminophen, and 20 μg / ml vitamin C, and has good specificity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com