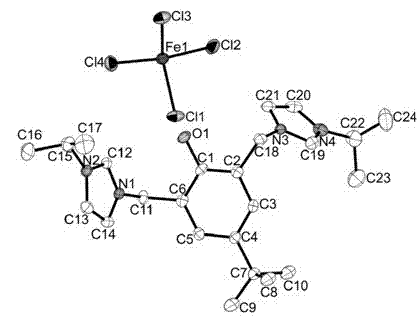
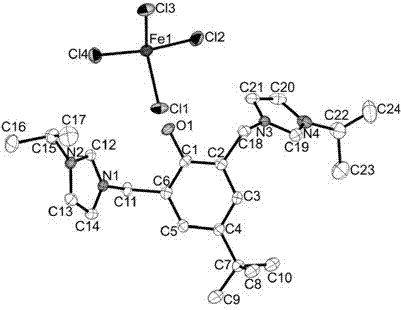
Ionized iron (III) coordination compound containing phenol-bridged imidazolium and application thereof
A biimidazolium salt and ionic technology is applied in the field of ionic iron complexes containing phenol bridged biimidazolium salts to achieve the effects of deepening understanding, strong electron donating ability and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] [O-4-C(CH 3 ) 3 -C 6 h 2 -2,6-di-{CH 2 [CH(NCHCHNR)]}]FeX 4 (R is isopropyl, X=Cl) synthesis.
[0039] Under anhydrous and oxygen-free conditions, in an argon atmosphere, a tetrahydrofuran (THF) solution of ferric chloride (0.1622g, 1mmol) was added to a THF suspension of phenol bridged imidazolium salt (0.4675g, 1mmol), Stir at room temperature for 1 hour, the solid in the reaction system gradually disappeared, and the color of the solution was yellowish brown. Remove the solvent in vacuo, extract the residue with THF, centrifuge, drain the solvent, add THF and hexane solvent in turn, and precipitate at 0-5 °C The crystal is the above-mentioned ionic iron (III) complex containing phenol bridged imidazolium salt, and the yield is 75%.
[0040] We carried out elemental analysis, melting point determination, Raman spectrum characterization and crystal structure determination of the product.
[0041] The elemental analysis and melting point determination results of ...
Embodiment 2
[0083] [O-4-C(CH 3 ) 3 -C 6 h 2 -2,6-di-{CH 2 [CH(NCHCHNR)]}]FeX 4 (For isopropyl, X=Br) synthesis.
[0084] Under anhydrous and oxygen-free conditions, in an argon atmosphere, a tetrahydrofuran solution of iron tribromide (0.2957g, 1mmol) and anhydrous and oxygen-treated sodium bromide (0.6585g, 6.4mmol) were sequentially added to the phenol bridge Biimidazole salt (0.4675g, 1mmol) in tetrahydrofuran suspension, stirred at room temperature for 2 hours, the solid in the reaction system gradually disappeared, the solution color was yellowish brown, the solvent was removed in vacuo, the residue was extracted with THF, centrifuged, and drained As a solvent, add THF and hexane solvent in sequence, and crystals are precipitated at 0-5° C., which is the above-mentioned ionic iron (III) complex containing phenol-bridged imidazolium salt, with a yield of 70%.
[0085] The product was characterized by elemental analysis, melting point determination and Raman spectroscopy.
[008...
Embodiment 3
[0090] [O-4-C(CH 3 ) 3 -C 6 h 2 -2,6-di-{CH 2 [CH(NCHCHNR)]}]FeX 4 (R is methyl, X=Cl) synthesis.
[0091] Under anhydrous and oxygen-free conditions, in an argon atmosphere, a tetrahydrofuran solution of ferric chloride (0.1622g, 1mmol) was added to a THF suspension of phenol bridged imidazolium salt (0.4955g, 1mmol), and stirred at room temperature After 1 hour, the solid in the reaction system gradually disappeared, and the color was yellowish brown. Remove the solvent in vacuo, extract the residue with THF, centrifuge, drain the solvent, add THF and hexane solvent successively, and precipitate crystals at 0-5 °C, which is The yield of the above-mentioned ionic iron (III) complex containing phenol bridged imidazolium salt is 76%.
[0092] The product was characterized by elemental analysis and Raman spectroscopy.
[0093] The elemental analysis results of the product are shown in the table below:
[0094] C:(%)
[0095] The complex was characterized by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com