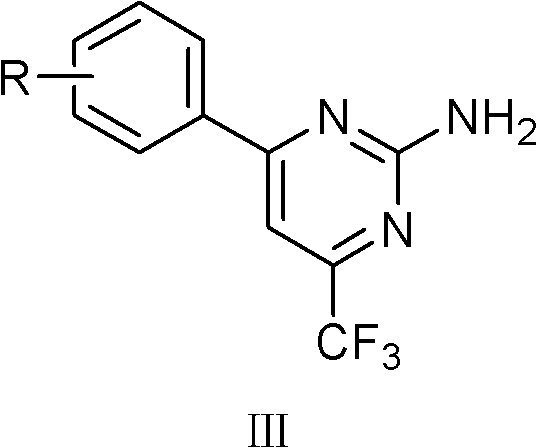
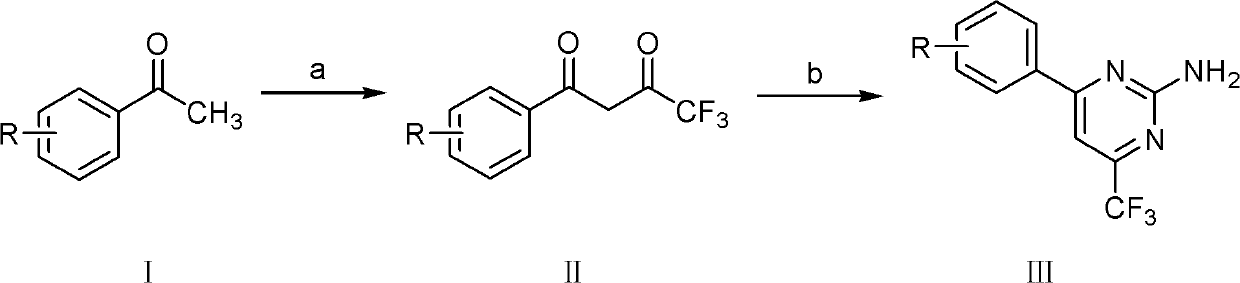
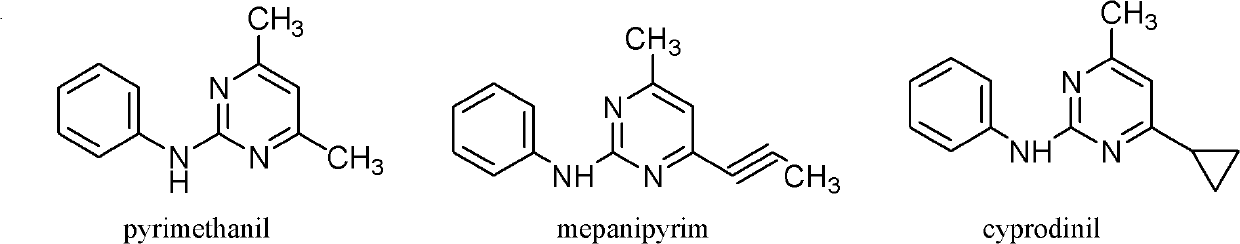
Trifluoromethyl-containing pyrimidinamine compound, preparation method thereof, and application of trifluoromethyl-containing pyrimidinamine compound used as bacteriacide
A technology of trifluoromethylpyrimidine ammonia compound and synthesis method, which is applied in the field of agricultural chemicals to achieve good bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Synthesis of Example 1.4-(2-fluorophenyl)-6-trifluoromethyl-2-aminopyrimidine (III-1)
[0018] Add 30 mL of methanol into a single-necked bottle, cut 0.2 mol of sodium metal into chips at 0-5°C, and add small amounts of it several times to prepare a sodium methoxide solution for later use. Add 60 mL of methanol, 0.1 mol of 2-fluoroacetophenone, and 0.15 mol of ethyl trifluoroacetate into the three-necked flask and stir evenly, then slowly add sodium methoxide dropwise into the three-necked flask, and control the temperature of the reaction solution at 30°C during the dropping process the following. After the dropwise addition, the temperature was raised to reflux for 1 h. The reaction solution was left to cool to room temperature, 50 mL of methanol was evaporated under reduced pressure, poured into 100 mL of ice water containing 30 mL of hydrochloric acid, stirred with a glass rod, a large amount of yellow solids precipitated, suction filtered, washed with water, and d...
example 2
[0028] Example 2. Preliminary screening of the bactericidal activity of compounds III-1~III-15
[0029] The mycelial growth rate method was used to measure the bactericidal activity of the target compound at 50 mg / L to the tested pathogenic fungi. Use a puncher with a diameter of 6 mm to make bacterial blocks from each pathogenic bacteria grown on the PDA medium, inoculate it on the poisonous PDA medium, cultivate it at 23°C±1°C for 3-5 days, and measure the colony by the cross method Diameter, according to (1) to calculate the inhibition rate.
[0030] Inhibition rate (%)=(blank control colony diameter-toxic medium colony diameter) / (blank control colony diameter-bacteria cake diameter)×100(1)
[0031] The bactericidal activity of the compound against 8 kinds of pathogenic bacteria was tested, and the test results are shown in Table 3. At a concentration of 50 μg / mL, compounds III-1, III-2, III-5, III-6, and III-10 showed high inhibitory activity against multiple pathogenic ...
example 3
[0034] Example 3. Compounds III-1~III-15 are tested for precision toxicity of Botrytis cinerea
[0035] The toxicity of 15 new compounds to Botrytis cinerea was measured by mycelium growth rate method, and the test results are shown in Table 4. The sample compounds were respectively configured into toxic media with concentrations of 100, 25, 6.25, 1.56, and 0.39 mg / L, a total of five gradients. Use cyprodinil as the control agent, set acetone as the blank control, and repeat three times. Using the statistical method of EXCEL software to calculate EC 50 , EC 80 value, to compare the toxicity of medicines.
[0036] EC from Table 3 50 and EC 80 It can be seen from the data that the toxicity of compounds III-1, III-10 and III-13 to Botrytis cinerea is higher than that of the control drug cyprodinil, especially the bactericidal activity of III-10 is significantly higher than that of other compounds.
[0037] Table 4 Compound III-1~III-15 is to the toxicity determination of Bo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com