Production method of medical biological material for human serum albumin
A technology of human serum albumin and its production method, which is applied in the field of preparation of porous sponge or film using human serum albumin, to achieve the effect of strong mechanical strength and strong ductility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
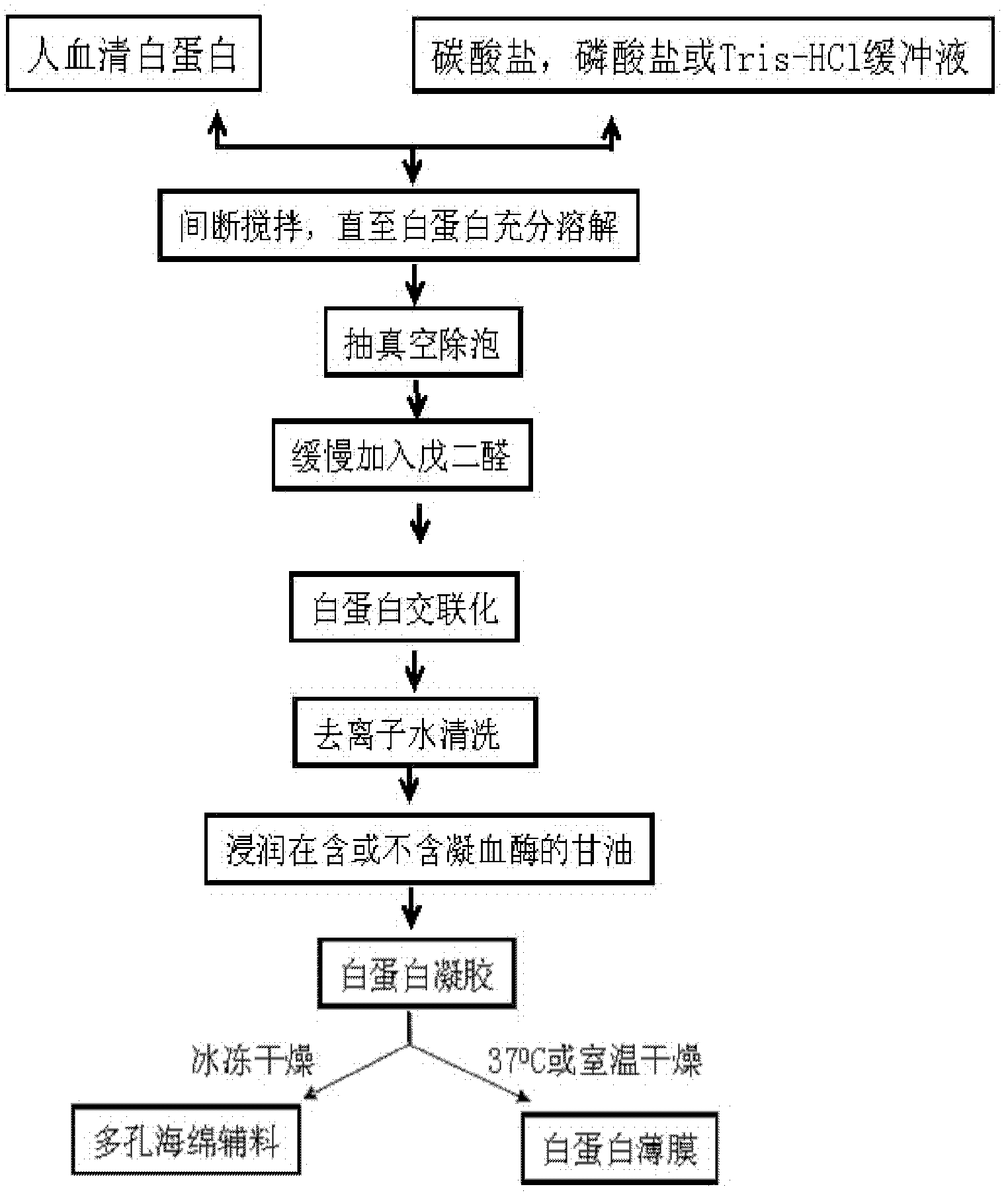
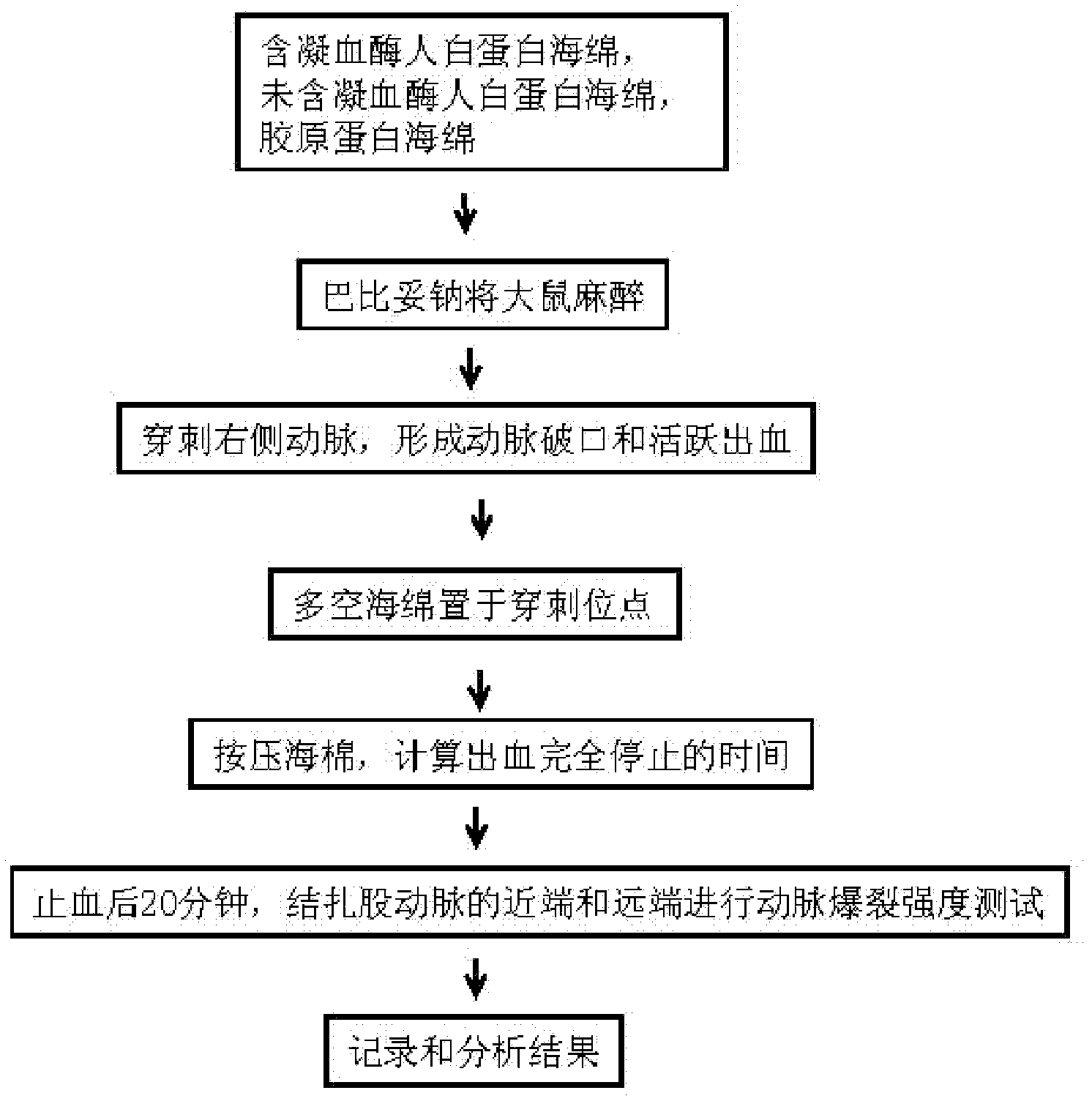
[0035] 0.5 g of recombinant human serum albumin (5%, w / v) was weighed and dissolved in 10 ml of sodium bicarbonate-sodium carbonate buffer (10 mM, pH 7.4) prepared from deionized water. Stir intermittently at 37°C for 1 hour to dissolve the protein. After the protein is completely dissolved and becomes a transparent liquid, use a vacuum pump for 30 minutes to quickly eliminate the bubbles in the solution. Slowly add 0.25% (v / v) glutaraldehyde to form a gel. The gel was washed 3 times with deionized water, 2 liters each time, for 3 hours to remove the buffer in the gel. Prepare 10% glycerol from deionized water, and infiltrate the albumin gel for 30 minutes. Place the albumin gel in a -70°C ultra-low temperature refrigerator to freeze for more than 4 hours, then put the frozen albumin gel into a freeze dryer, and vacuum-dry it for 24 hours to make a porous sponge without thrombin . The albumin porous sponge was sealed and stored dry at 4°C. This albumin porous sponge has ex...
Embodiment 2
[0037] 0.5 g (5%, w / v) of plasma-derived human serum albumin was weighed and dissolved in 10 ml of sodium bicarbonate-sodium carbonate buffer (10 mM, pH 7.4) prepared from deionized water. Stir intermittently at 37°C to dissolve it. After the protein is completely dissolved and becomes a transparent liquid, use a vacuum pump to pump air for 30 minutes to quickly eliminate the bubbles in the solution. Slowly add 0.25% (v / v) glutaraldehyde to form a gel. The gel was washed 3 times with deionized water, 2 liters each time, for 3 hours to remove the buffer in the gel. Prepare 10% glycerol from deionized water, and infiltrate the albumin gel for 30 minutes. Place the albumin gel in a -70°C ultra-low temperature refrigerator to freeze for more than 4 hours, then put the frozen albumin gel into a freeze dryer, and vacuum-dry it for 24 hours to make a porous sponge without thrombin . The performance of this sponge is slightly worse than that of the albumin sponge derived from gene ...
Embodiment 3
[0039] Weigh 1.0 g (10%, w / v) of human serum albumin produced by fermentation of genetically engineered yeast, and dissolve it in 10 ml of sodium phosphate buffer (10 mM, pH 7.4) prepared from deionized water. Stir intermittently at 37°C for 1 to dissolve the protein. After the protein is completely dissolved and becomes a transparent liquid, use a vacuum pump to pump air for 30 minutes to quickly eliminate the bubbles in the solution. Slowly add 0.125% (v / v) glutaraldehyde to form a gel. The gel was washed 3 times with deionized water, 2 liters each time, for 3 hours to remove the buffer in the gel. Prepare 10% glycerol from deionized water, and infiltrate the albumin gel for 30 minutes. Place the albumin gel in a -70°C ultra-low temperature refrigerator to freeze for more than 4 hours, then put the frozen albumin gel into a freeze dryer, and vacuum-dry it for 24 hours to make a porous sponge without thrombin . This albumin sponge has poor water absorption (12 times) with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com