Potential of hydrogen (pH) response random copolymer based on poly-beta amino ester and preparation method and application thereof
A technology of random copolymers and amino esters, which is applied in the direction of medical preparations of non-active ingredients, drug combinations, antineoplastic drugs, etc., can solve the problems of drug side effects, slow process, and aggravated burst release, and achieve high load The effect of the dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
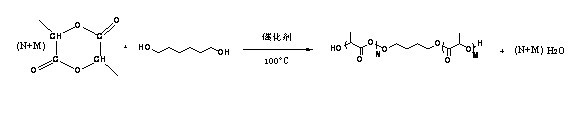
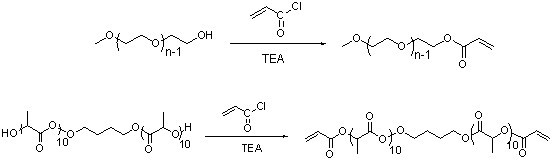
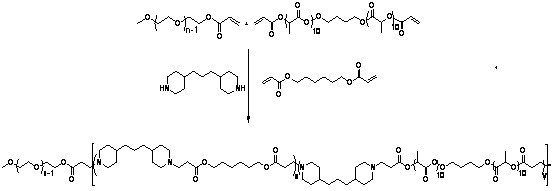
[0092] (1) Synthesis of HO-PLA-OH (A:B:C=100:5:1, A represents the monomer lactide, B represents the initiator 1,4-butanediol, C represents the catalyst Sn(Oct) 2 , the ratio is mass percentage, the same below). Add a stirring bar and monomeric lactide (20 g, 93.9 parts) into a 100 mL reaction bottle with a stirring bar, seal it and pump-ventilate three times, under the protection of argon, add the solvent toluene (40 mL ) and catalyst Sn(Oct) 2 (0.3 mg, 1.41 parts) and the initiator 1,4-butanediol (1 g, 4.69 parts). After freezing with liquid nitrogen, vacuumize-heat-inflate three times, and react in an oil bath at 100 °C for 24 h under the protection of argon. After the reaction was completed, the obtained solution was evaporated under reduced pressure to remove toluene, and then the polymer was dissolved in chloroform to obtain a solution, which was slowly added to 300 mL (equivalent to 10 times the volume of the solution) of refrigerated methanol / water (1:1, v / v), wash...
Embodiment 2
[0098] (1) Synthesis of HO-PLA-OH (A:B:C=100:15:3, A represents the monomer lactide, B represents the initiator 1,4-butanediol, C represents the catalyst Sn(Oct) 2 , the ratio is mass percentage, the same below). Add a stirrer and monomeric lactide (10 g, 91.32 parts) into a 100 mL reaction bottle with a stirrer, seal it and pump-ventilate three times, under the protection of argon, add the solvent toluene (40 mL ) and catalyst Sn(Oct) 2 (0.15 mg, 1.37 parts) and the initiator 1,4-butanediol (0.8 g, 7.31 parts). After freezing with liquid nitrogen, vacuumize-heat-inflate three times, and react in an oil bath at 95 °C for 30 h under the protection of argon. After the reaction was completed, the obtained solution was evaporated under reduced pressure to remove toluene, and then the polymer was dissolved in chloroform to obtain a solution, which was slowly added to 300 mL (equivalent to 10 times the volume of the solution) of refrigerated methanol / water (1:1, v / v), washed twi...
Embodiment 3
[0103] (1) Synthesis of HO-PLA-OH (A:B:C=300:5:1, A represents the monomer lactide, B represents the initiator 1,4-butanediol, C represents the catalyst Sn(Oct) 2 , the ratio is mass percentage, the same below). Add a stirrer and monomeric lactide (30 g, 98.04 parts) into a 100 mL reaction bottle with a stirrer, seal it and pump-ventilate three times, under the protection of argon, add the solvent toluene (40 mL ) and catalyst Sn(Oct) 2 (0.1 mg, 0.33 parts) and the initiator 1,4-butanediol (0.5 g, 1.63 parts). After freezing with liquid nitrogen, vacuumize-heat-inflate three times, and react in an oil bath at 105 °C for 20 h under the protection of argon. After the reaction was completed, the obtained solution was evaporated under reduced pressure to remove toluene, and then the polymer was dissolved in chloroform to obtain a solution, which was slowly added to 300 mL (equivalent to 10 times the volume of the solution) of refrigerated methanol / water (1:1, v / v), washed twic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Critical micelle concentration | aaaaa | aaaaa |
| Survival rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com