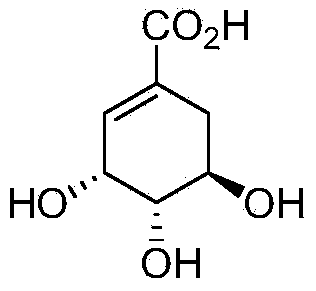
Synthesis method of shikimic acid
A synthesis method and technology of shikimic acid are applied in chemical instruments and methods, preparation of carboxylate, preparation of oxygenated compounds, etc., can solve problems such as low optical purity, low total yield, complicated operation, etc., and achieve a simple and easy method. performance, cost reduction, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
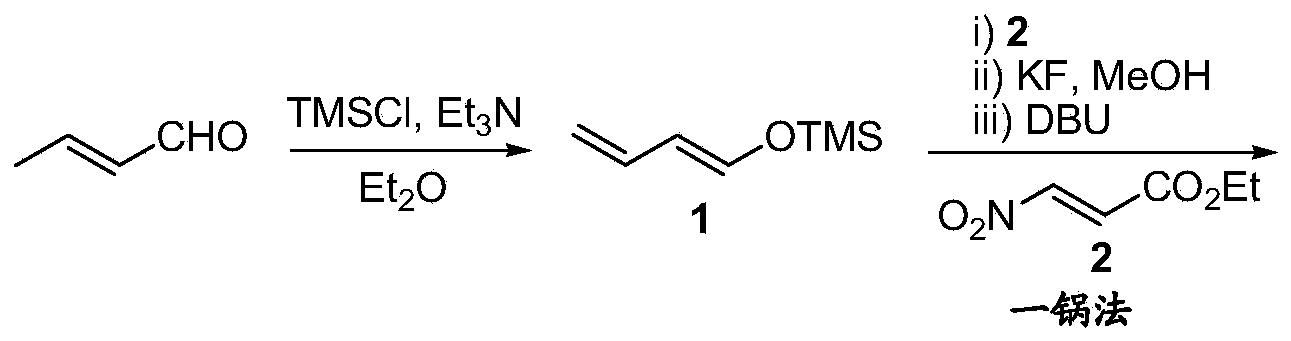
[0040] Add dry triethylamine (33mL, 240mmol) and anhydrous zinc chloride powder (0.9g, 6mmol) into a 250mL three-necked flask, stir the reaction system at room temperature for 1 hour, then add crotonaldehyde (14g, 200mmol) 100mL ether solution, after stirring for 5 minutes, trimethylchlorosilane (29mL, 230mmol) was added dropwise, and the dropwise addition was completed within half an hour. The reaction system was refluxed overnight and cooled to room temperature, then diluted with 100 mL of dry petroleum ether, the organic liquid was filtered through a short (4 cm) alumina column, the filtrate was concentrated, and the colorless liquid was obtained by distillation under reduced pressure (26 g, yield: 92% ), boiling point: 131°C; 1 H-NMR (CDCl 3 ,400MHz):δ6.54(d,J=11.9Hz,1H),6.22(ddd,J=10.3,6.5,4.3Hz,1H),5.73(dd,J=11.9,11.1Hz,1H),4.99( m,1H), 4.83(d,J=10.3Hz,1H),0.22(s,9H). It shows that the colorless liquid is 1,3-butadienyl trimethylsilyl ether shown in structural formula...
Embodiment 2
[0042] Add 1,3-butadienyl trimethylsilyl ether (15 g, 105 mmol) shown in structural formula 1 and 14.5 g (100 mmol) of 3-nitro-acrylic acid ethyl alcohol shown in structural formula 2 in a 250 mL three-necked flask Esters, the reaction system was vigorously stirred at room temperature for 4 hours (track the reaction with gas chromatography), after the reaction was complete, stop stirring and add 50mL methanol to dissolve, then add potassium fluoride (8.7g, 150mmol) and continue stirring for 2 hours, stop stirring and add 50mL tetrahydrofuran and 1,8-Diazabicyclo[5.4.0]undec-7-ene (21.2 g, 140 mmol) was reacted at room temperature for 3 hours and the reaction was stopped. The reaction system was quenched by adding 50 mL of water, extracted three times with ethyl acetate (50 mL), and the combined organic phases were first washed with 10% aqueous hydrochloric acid (20 mL) with a concentration of 10% by mass, and then washed with saturated aqueous sodium bicarbonate and saturated b...
Embodiment 3
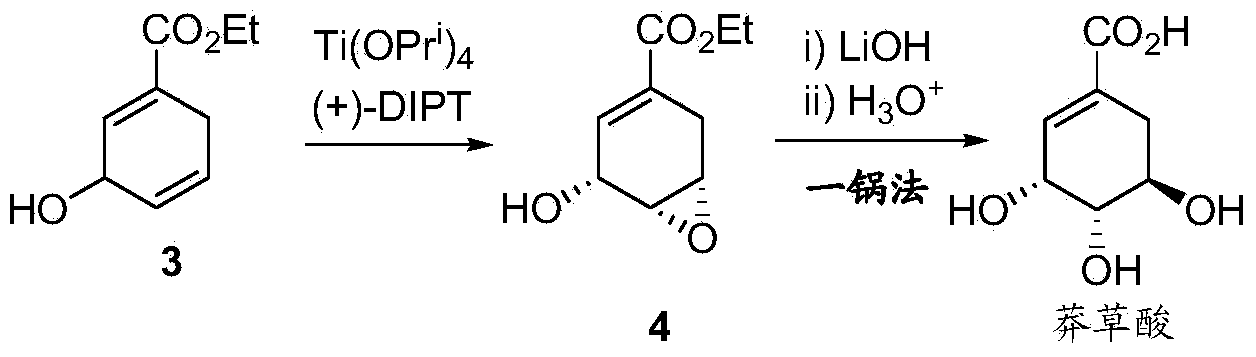
[0044] Except that 85mL of ethanol was used instead of 50mL of methanol in Example 2 and potassium fluoride was added to react at 50°C for 10 hours, the rest of the operating steps were the same as in Example 2 to obtain 3-hydroxyl-1,4-cyclohexane di Ethyl-1-carboxylate, yield 61%, product characterization data with embodiment 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com