Amphoteric ionic polymer clay stabilizer and preparation method
A clay stabilizer and zwitterion technology, applied in the field of zwitterionic polymer clay stabilizer and preparation, can solve the problems of high temperature resistance, formation loss and shear resistance and high cost, and achieve good temperature and salt resistance and shear resistance. The effect of improving the stability, increasing the rigidity of the molecule
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of NAO monomer
[0031] Add 42.3g of oleic acid to the reactor, slowly add 10.2g of phosphorus trichloride under stirring, the feeding temperature is controlled at 10~15℃, after the addition, the temperature is raised to 55℃, after 4h, the lower layer liquid is separated and the upper layer liquid The unreacted phosphorus trichloride was removed by evaporation, yielding 98.8% oleyl chloride; 18.7g of the oleyl chloride obtained in the previous step was diluted with dichloromethane solvent and put into a constant pressure dropping funnel for use, and then placed in 250ml of dry trichloride. Add 4g of allylamine to the neck flask, dilute with dichloromethane solvent, then add 7.6g of triethylamine and 0.02g of hydroquinone, slowly add the ready-to-use oleyl chloride solution in an ice bath with constant stirring, and control the drop for 1h Finished; reaction at room temperature for 5h after dripping, the reaction solution was washed with water, acid w...
Embodiment 2
[0032] Example 2: Preparation of AM / NaAA / DMDAAC / NAO quaternary polymer
[0033] First weigh AA and dilute with 10g deionized water according to the ratio in Table 1, slowly add sodium hydroxide in an ice bath, and cool to room temperature; add the NAO prepared above into a 250ml three-neck flask, and then add OP-10 to emulsify Add AM and DMDAAC after emulsification is complete, then add NaAA solution, adjust the pH to 8 with NaOH solution, and blow nitrogen for 20 minutes; then add initiator sodium bisulfite solution, then add persulfuric acid Ammonium solution, nitrogen gas for 10min, at a temperature of 45 o React for 10h at C; finally wash five times with absolute ethanol, crush, 40 o C constant temperature drying to prepare AM / NaAA / DMDAAC / NAO quaternary polymer.
[0034]
Embodiment 3
[0035] Example 3: Structural characterization of AM / NaAA / DMDAAC / NAO quaternary polymer
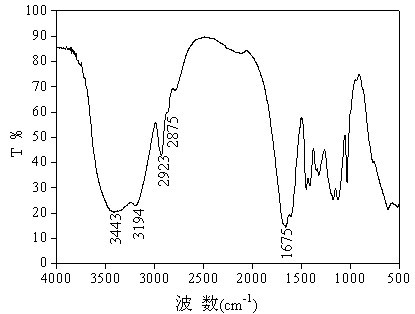
[0036] The infrared spectrum of the quaternary polymer AM / NaAA / DMDAAC / NAO synthesized in Example 2 is as follows figure 1 Shown. The figure shows the infrared spectrum of the polymer at 3443 cm -1 A strong absorption peak is generated at the O-H stretching vibration at 3194 cm -1 There is a strong absorption peak corresponding to the N-H stretching vibration at 2923 cm -1 And 2875cm -1 The strong absorption peak is -CH 3 And -CH 2 -The stretching vibration at 1675cm -1 It is the stretching vibration of -C=O, which proves that there is an amide structure in the polymer molecule, and the product is AM / NaAA / DMDAAC / NAO quaternary polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Apparent viscosity | aaaaa | aaaaa |
| Apparent viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com