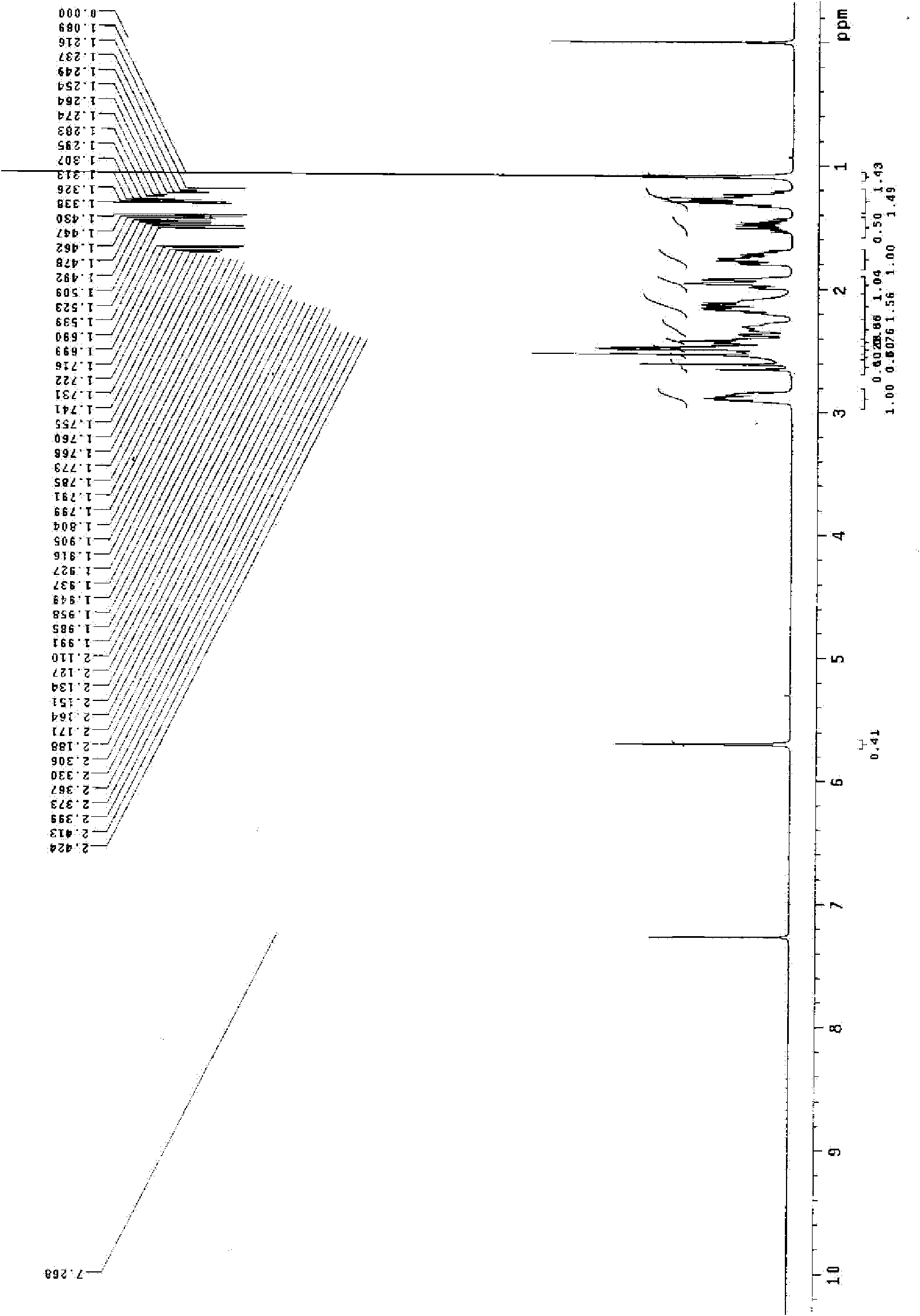
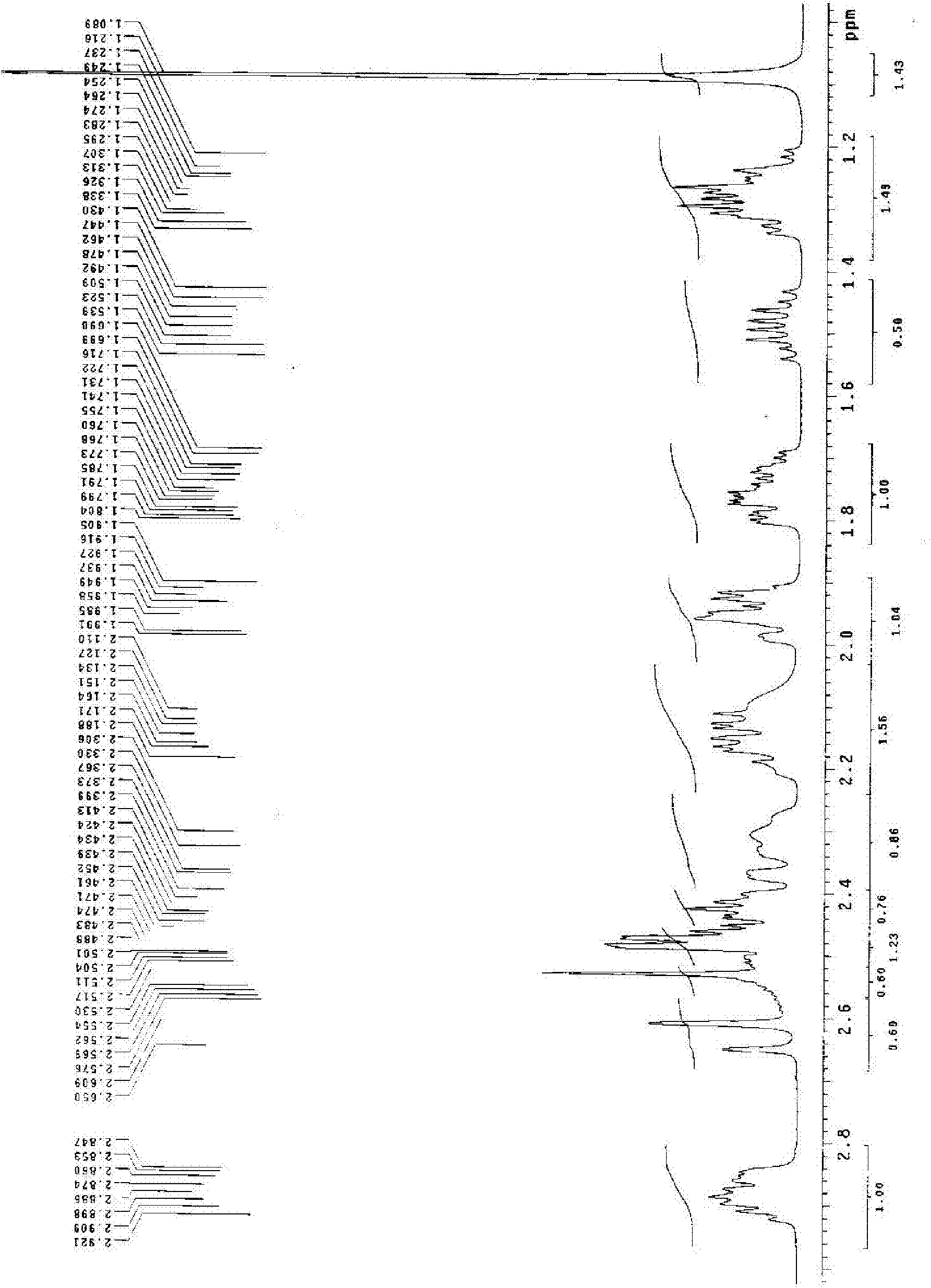
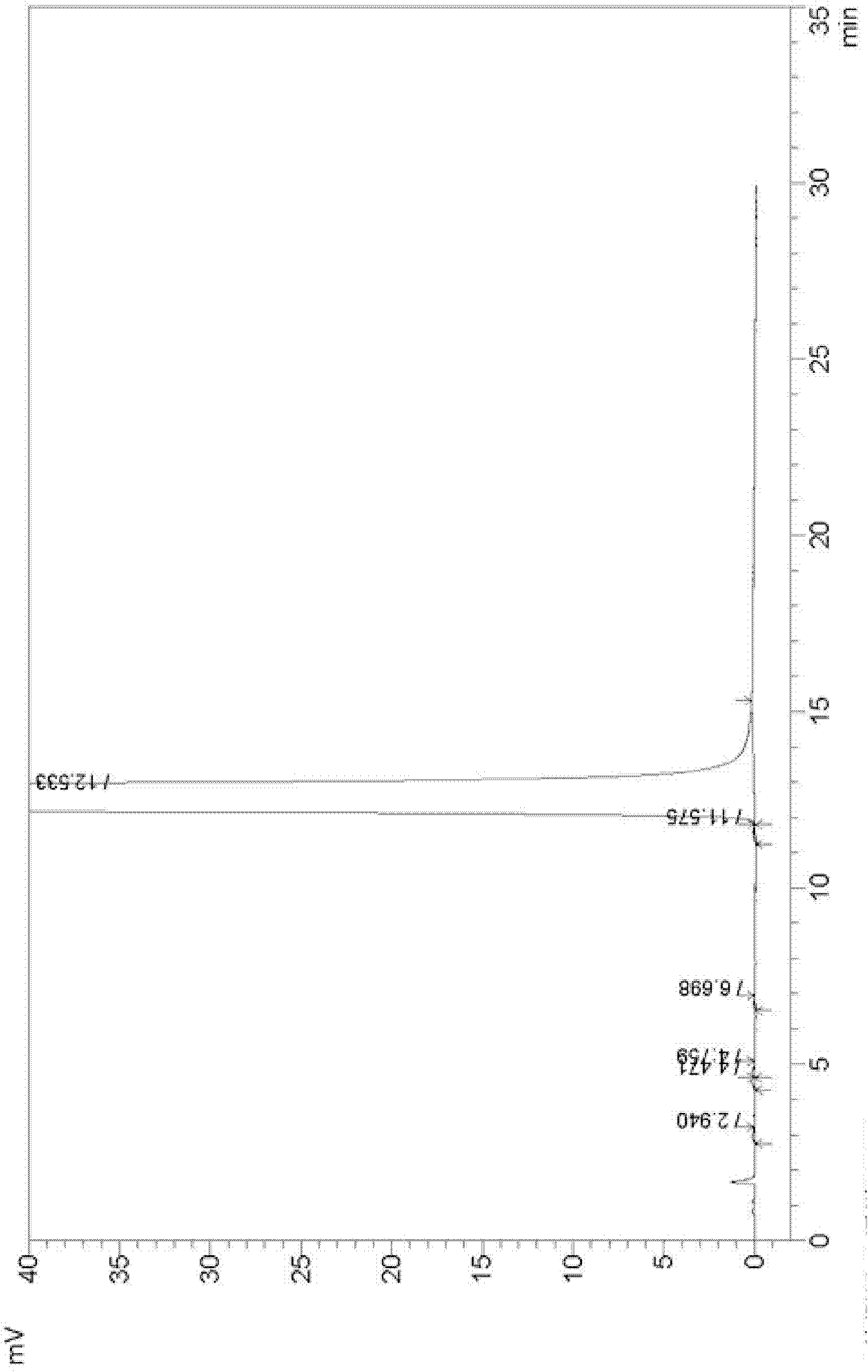
Preparation method for dienogest
A technology for dienogest and compound, which is applied in the field of compound preparation and achieves the effects of strong alkalinity, less side reactions and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] In this embodiment, the R base is And n=2.
[0045] (1) Preparation of formula 12 compound
[0046] In a 1L round bottom flask, add 500 mL of cyclohexane, 163 g of triethyl orthoformate (183 mL, 1.10 mol), 96.2 g of ethylene glycol (86.3 mL, 1.55 mol) and 0.9 g of p-toluenesulfonic acid (4.70 mmol), and stir Reflux reaction, fractional distillation of ethanol-cyclohexane azeotrope at normal pressure, additional cyclohexane to keep the volume of the reaction solution basically constant. After completion of the reaction, 5g of potassium carbonate was added to the system, and under reduced pressure distillation, the fraction above 130°C was collected under 10mmHg, which was a colorless oily liquid to obtain 85g of active ester (chemical name: 2,2'-(1,2-ethylene Dioxy)-bis-1,3-dioxolane, hereinafter referred to as active ester), the yield is about 80%, ρ=1.03g / mL.
[0047] In a 3L round bottom flask, under nitrogen protection, add 151g of estro-4,9-diene-3,17-dione, add...
Embodiment 2
[0056] The base R is And n=2.
[0057] (1) Preparation of formula 12 compound
[0058] With embodiment 1.
[0059] (2) Preparation of formula 14 compound
[0060] In a 500mL single-necked bottle, add 15g of the compound of formula 12, 150mL of isopropyl ether, stir to dissolve, add 13mL of acetonitrile, protect with nitrogen, and add 48mL of tetrahydrofuran solution of lithium hexamethyldisilazide dropwise at an external temperature of -20 to 0°C (the content of lithium hexamethyldisilazide is 2mol / L), react at the same temperature for 3 hours after dropping, add 60mL of water, adjust the pH to about 2 with 1N hydrochloric acid, extract three times with ethyl acetate, and combine the acetic acid The ethyl ester phase was washed with saturated brine, dried, and concentrated under reduced pressure to obtain a brown solid. Recrystallization with ethyl acetate gave 15 g of a light yellow solid with a yield of 88.2% and a purity of 99.3% by HPLC.
[0061] (3) Refining of form...
Embodiment 3
[0064] The base R is wherein R' is a methyl group.
[0065] (1) Preparation of formula 12 compound
[0066] In a 1L round bottom flask, add 540mL of anhydrous methanol and 50g of estro-4,9-diene-3,17-dione, stir, and under the protection of nitrogen, add 120mL of 20% hydrochloric acid / methanol dropwise, and continue After reacting for three hours, the reaction was quenched by adding saturated aqueous sodium bicarbonate solution, part of the solvent was distilled off, and a white solid was precipitated. After suction filtration, the filter cake was recrystallized from ethyl acetate to obtain 54 g of white solid, with a yield of about 97%.
[0067] (2) Preparation of formula 14 compound
[0068] In a 500mL single-necked bottle, add 15g of the compound of formula 12, 150mL of isopropyl ether, stir to dissolve, add 13mL of acetonitrile, nitrogen protection, and at an external temperature of -40~-80°C, add dropwise 95mL of lithium hexamethyldisilazide tetrahydrofuran solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com