Carbazole hemicyanine fluorescent dye and application thereof
A fluorescent dye, carbazole-based technology, applied in the field of carbazole-based semicyanine fluorescent dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Synthesis of Compound Hcaz
[0068] The synthesis flow method of compound Hcaz is as follows:
[0069]
[0070] (1) The synthesis of 2,3,3-trimethyl-3H-indoline (compound 1) follows the fisher indole synthesis method:
[0071] Weigh 54g (0.5mol) of phenylhydrazine and add it to a 250mL two-necked bottle, slowly add 43g (0.5mol) of 3-methyl-2-butanone dropwise under stirring, heat to 70~80°C, react for 4 hours, and separate to remove the water layer , the aqueous layer was extracted with ether, combined with the ether layer, dried and filtered with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 70 g of crude hydrazone with a yield of 80%.
[0072] Mix 70g (0.4mol) of the crude hydrazone from the previous step with 150mL of glacial acetic acid, react in an oil bath at 90°C for 3 hours, cool to room temperature, neutralize the water layer with saturated aqueous sodium carbonate solution until neutral, separate the water ...
Embodiment 2
[0085] Synthesis of Compound Qcaz
[0086] The synthetic method of compound Qcaz is as follows:
[0087]
[0088] (1) Synthesis of N-ethyl-3,6-dialdehyde carbazole (compound 5):
[0089] Accurately measure 18 mL of dry DMF with a pipette and place it in a dry 100 mL single-necked flask, place the single-necked flask in an ice-water bath, and drop 15 mL of phosphorus oxychloride into the single-necked flask. Analytical balance Accurately weigh N-ethylcarbazole (2.8g, 14.3mmol) dissolved in 40mL of chlorobenzene, place in a constant pressure dropping funnel, and install a constant pressure dropping funnel. After 0.5h, the chlorobenzene solution of N-ethylcarbazole was slowly dropped into a one-necked flask, and stirred and reacted in an oil bath at 90~95°C for 60h. After the reaction was completed, cool, adjust the pH to neutral with 10% sodium bicarbonate solution, extract with chloroform, dry with anhydrous sodium sulfate, remove the solvent under reduced pressure, and recr...
Embodiment 3
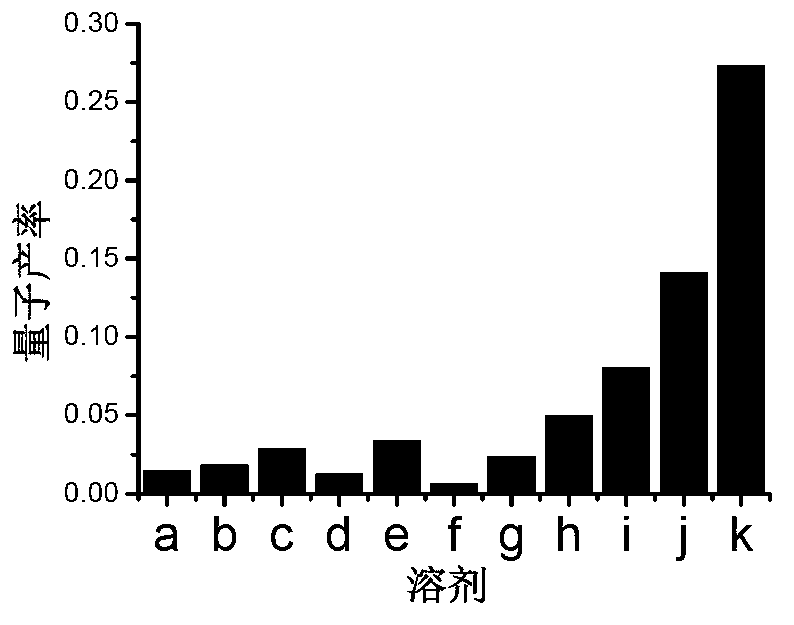
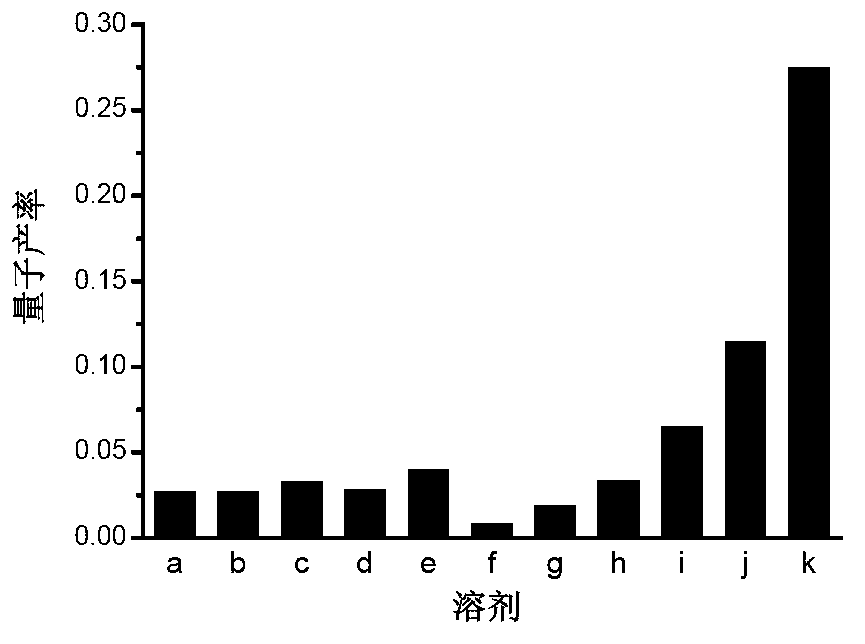
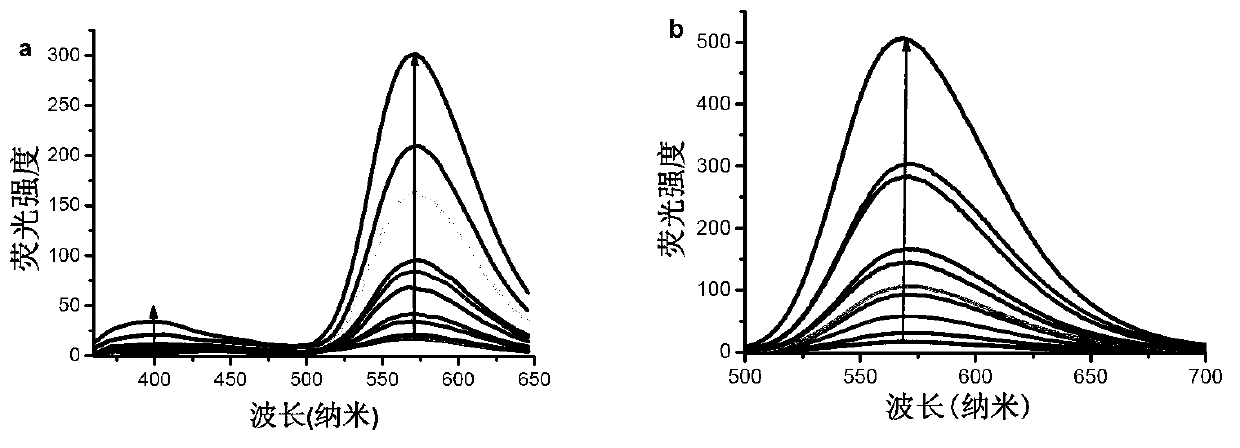
[0097] Viscosity test of compounds Qcaz and Hcaz
[0098] The preparation concentration is 1×10 -3 The DMSO solution of the compounds Qcaz and Hcaz of M, accurately measure 10 μL of the solution and add it to 10mL glycerol-water solution, ultrasonic for 10 minutes, remove the air bubbles and let it stand for 1 hour, and measure it on the ultraviolet spectrophotometer and fluorescence photometer. Absorption and emission spectra. The excitation wavelength chosen was 330 nm. The instruments used are ultraviolet-visible spectrophotometer, model: Hp8453; fluorescence spectrophotometer, model: FP-6500, fluorescence lifetime tester: Horiba Jobin Yvon Fluoromax-4p. The excitation source of the two-photon excitation fluorescence spectrum is a mode-locked femtosecond titanium sapphire exciter, and the fluorescence detection is detected by a Leica confocal microscope fluorescence detection system.
[0099] Wherein, the glycerol-ethanol solution includes glycerol, ethanol and V 甘油 :V ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com