Preparation method of low-abundance low-molecular-weight protein-rich material
A low-molecular-weight, low-abundance technology, used in the preparation of test samples, material inspection products, biological testing, etc., can solve problems such as degradation, interference effects, and easy loss of target proteins, and achieve the effect of avoiding loss and preventing degradation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of porous silicon
[0019] Take the P-type boron-doped silicon wafer (100) crystal form, fix it in the electrolytic cell, add 0.5ml ethanol, 2-3ml hydrofluoric acid with a mass concentration of 40% as the electrolyte, use the silicon wafer as the anode and the platinum electrode as the cathode , Carry out DC electrolytic etching and stripping, set the current intensity to 10~80mA, the etching time is 5 minutes, the porous silicon layer after etching and stripping is washed with ethanol and ultrasonically crushed for 15 minutes, and then dried with nitrogen , The pore size of the obtained porous silicon particles is 5-20nm;
[0020] The above porous silicon particles are subjected to ozone oxidation treatment for 20 minutes to form surface silicon-oxygen bonds;
[0021] The oxidized porous silicon particles were immersed in a mixed solution of 1% aminosilylation reagent and ethanol solvent for 1 hour, and then dried in an oven at 110°C for 15 minutes. The...
Embodiment 2
[0023] Example 2 Processing and testing of insulin samples
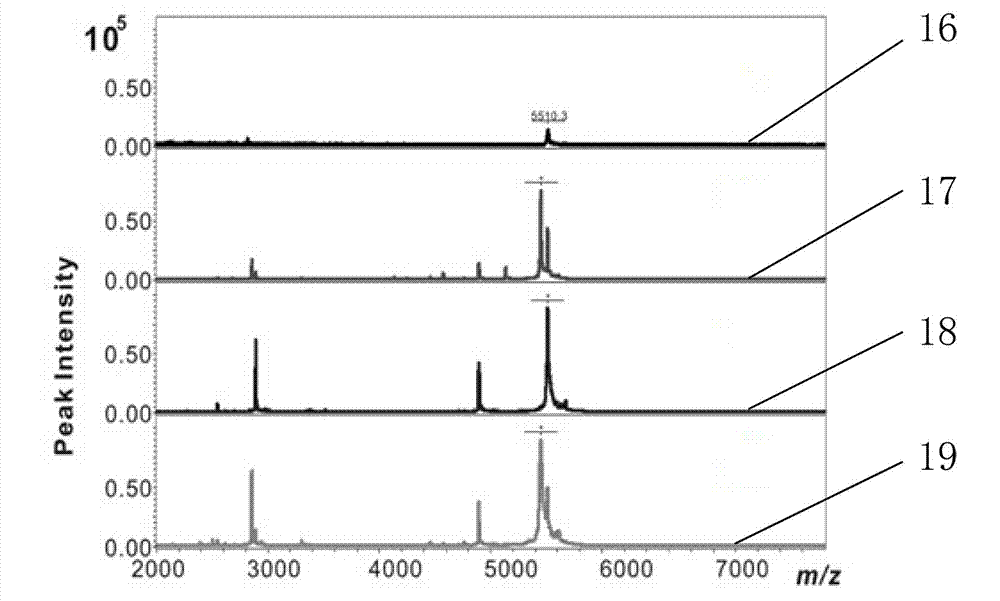
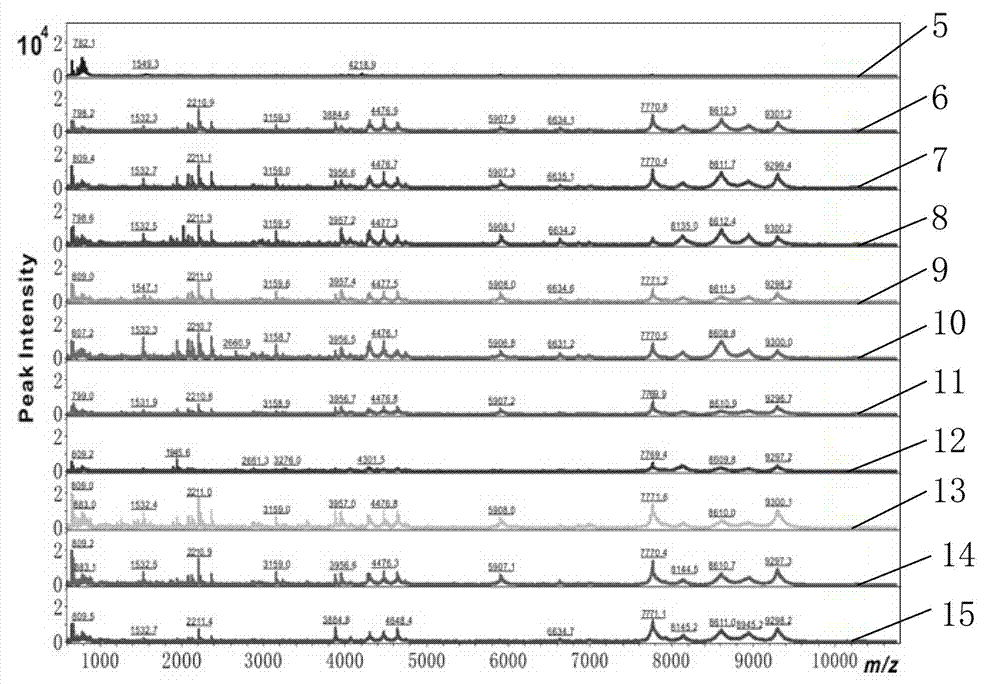
[0024] In this example, the porous silicon particles modified with undecylenic acid with a porosity of 26% were used as the enrichment material to directly detect low-concentration insulin samples. The specific steps are as follows:
[0025] 1. Sample adsorption
[0026] The insulin sample in this example is a medical human injection, and the insulin injection is diluted with physiological buffer PBS to 1.0 mg·mL -1 Then as the solution to be tested.
[0027] Adsorption: Take four centrifuge tubes, labeled 1, 2, 3, 4, and add 50 uL of the test solution to each, then add 3 mg of unetched silicon powder to centrifuge tube 2, and 3 mg to centrifuge tube 3. For porous silicon particles with a porosity of 26%, 3 mg of porous silicon particles with a porosity of 26% modified by undecylenic acid were added to the centrifuge tube 4, and the four centrifuge tubes were shaken and incubated for 1 h at room temperature.
[0028] Separati...
Embodiment 3
[0034] Example 3 Processing and testing of patient serum samples
[0035] In this embodiment, the porous silicon particles modified with undecylenic acid with a porosity of 26% are used as the enrichment material to directly detect and analyze the patient's serum sample. The specific steps are as follows:
[0036] 1. Sample adsorption
[0037] The serum sample in this example was obtained from a hospital. The preparation of the serum sample was performed by those skilled in the medical field. Among them, 5-9 are the serum of rectal cancer patients, and 10-14 are the serum of non-cancer patients. The prepared serum samples are stored in- 80 ℃ ultra-low temperature refrigerator.
[0038] Adsorption: Dilute the serum sample 10 times with physiological buffer PBS and add 50 uL to a centrifuge tube, then add 3 mg of undecylenic acid modified porous silicon particles with a porosity of 26% to obtain a suspension Incubate with shaking for 1 h at room temperature.
[0039] Separation: The abo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com