Method for pretreating coal chemical industry production waste water and recycling resources of waste water
A technology for producing waste water and recycling methods, applied in the field of water treatment, can solve the problems of poor biochemical feasibility of waste water, high treatment cost, complicated operation, etc., and achieve the effects of easy industrialization and application, obvious economic benefits and obvious environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
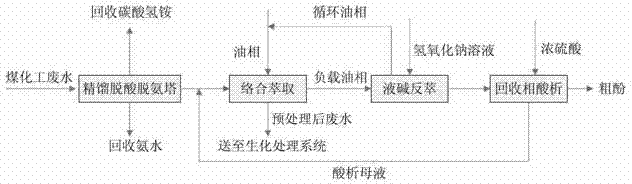
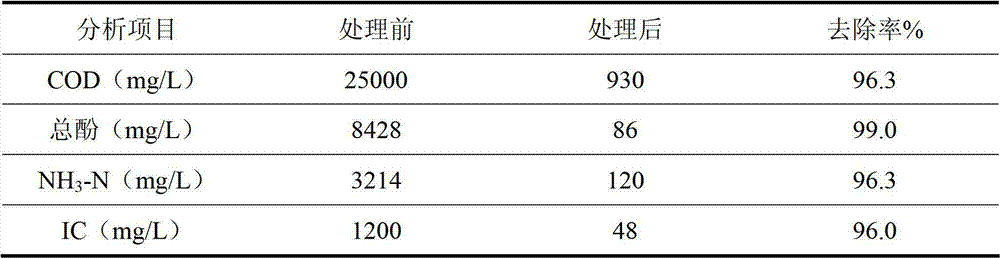
[0032] The waste water used in this example is taken from a blue carbon manufacturer in Yulin, Shaanxi. The water quality is as follows: COD is 25000mg / L, total phenol concentration is 8428mg / L, ammonia nitrogen concentration is 3214mg / L, IC (inorganic carbon) concentration is 1200 mg / L , pH value is 9.
[0033] Take 500mL of coal chemical wastewater and send it to the rectification deacidification and deamination tower. The wastewater in the tower is heated to boiling. The ammonia and carbon dioxide in the wastewater are transferred from the liquid phase to the gas phase. The mixed gas extracted from the top of the tower is condensed in two stages. The primary condensation temperature was controlled at 45° C., and the reflux ratio was controlled to be 5 to obtain a distillate with a volume of 2.5 mL, wherein the mass concentration of ammonia water was 15%. The secondary condensation temperature is controlled at 20°C, so that uncondensed ammonia and carbon dioxide directly rea...
Embodiment 2
[0040] Wastewater source is the same as embodiment 1.
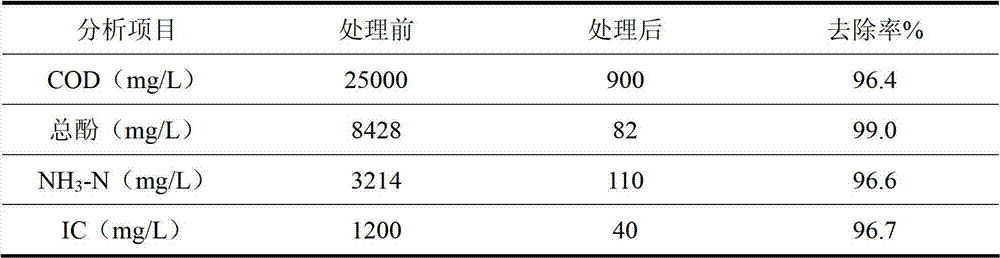
[0041] Take 500mL of coal chemical wastewater and send it to the rectification deacidification and deamination tower. The wastewater in the tower is heated to boiling. The ammonia and carbon dioxide in the wastewater are transferred from the liquid phase to the gas phase. The mixed gas extracted from the top of the tower is condensed in two stages. The primary condensation temperature is controlled at 35°C, and part of the condensate is sent to the rectification tower as a reflux liquid with a reflux ratio of 10, and the other part of the condensate is directly withdrawn, with a volume of 7.5mL and a concentration of ammonia water of 20%. The secondary condensation temperature is controlled at 30°C, so that uncondensed ammonia and carbon dioxide directly react to form solid ammonium bicarbonate, and the wet weight of the obtained solid is 4.2g.
[0042]Measure 30mL extractant TOPO and 70mL n-octanol, mix well to form oil ...
Embodiment 3
[0048] The waste water used in this example is taken from a blue carbon manufacturer in Inner Mongolia. The water quality is as follows: COD is 28560mg / L, total phenol concentration is 8300mg / L, ammonia nitrogen concentration is 7100mg / L, IC (inorganic carbon) concentration is 2500 mg / L, The pH value is 9.5.
[0049] Take 800mL of coal chemical wastewater and send it to the rectification deacidification and deamination tower. The wastewater in the tower is heated to boiling. The ammonia and carbon dioxide in the wastewater are transferred from the liquid phase to the gas phase. The mixed gas extracted from the top of the tower is condensed in two stages. The primary condensation temperature is controlled at 40°C. Part of the condensate is sent to the rectification tower as reflux liquid with a reflux ratio of 8, and the other part of the condensate is directly withdrawn with a volume of 8mL and a concentration of ammonia water of 18%. The secondary condensation temperature is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com