Preparation method of lacosamide
A lacosamide and amidation reaction technology, which is applied in the preparation of carboxylic acid amide optical isomers, bulk chemical production, organic chemistry, etc., can solve the problem of high cost, complicated operation, and unsatisfactory optical purity of lacosamide. and other problems to achieve the effect of eliminating racemization and improving the overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
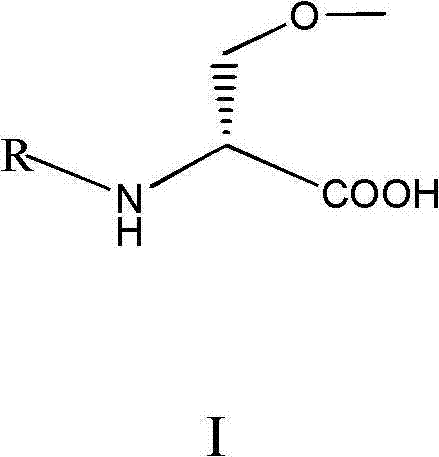
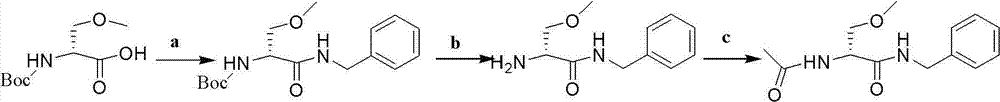
[0028] The invention relates to an improvement of a synthesis method of lacosamide for treating epilepsy and neuropathic pain.
[0029] The specific implementation steps are as follows:
[0030]
[0031] Process 1
[0032] The characteristics of reaction step a in process flow 1 are:
[0033] 1) Solvent selection
[0034] Alcohols (isopropanol, ethanol, methanol, etc.), halogenated alkanes (methylene chloride, chloroform, etc.), pyridine furans (dihydropyridine, tetrahydrofuran, etc.), toluene, dimethyl sulfoxide (DMSO), N, N -Dimethylformamide (DMF) etc. can be used as the reaction solvent, preferably dry dichloromethane as the solvent, after testing, dichloromethane is used as the solvent, the degree of product racemization is significantly reduced, and the boiling point is low, easy to be used remove.
[0035] 2) Choice of temperature
[0036] The present invention starts from the raw materials, and the intermediates and final products of each step have chirality, s...
Embodiment 1
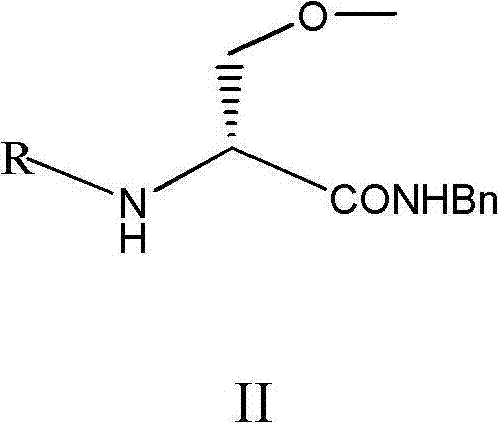
[0063] Synthesis of (R)-N-benzyl-2-N-Boc-amino-3-methoxypropionamide (compound of formula II)
[0064] In the reaction flask, dissolve 10g of (R)-2-N-Boc-amino-3-methoxypropionic acid in 80ml of dry tetrahydrofuran, and control the temperature of the reaction solution at -15~-10°C; at -10~ Add 5.6g isobutyl chloroformate and 6.2g N-methylmorpholine at -5°C, stir for 1.5h, then add benzylamine ( 5.89g) solution, slowly warming up to 25-30°C within 0.5h to react for 2.0h, HPLC method to detect whether the reaction is complete. After the reaction is finished, wash the reaction solution with 80ml of water, 80ml of 1mol / L hydrochloric acid, 80ml of saturated sodium bicarbonate solution and 80ml of water successively, separate the lower organic phase and concentrate to dryness to obtain an oil, then add 80ml of diethyl ether: n-hexane=1 : 1 mixed solvent, cooled and stirred in ice water, crystallized, and suction filtered to obtain 9.2 g of white powdery solid, with a yield of 86.0...
Embodiment 2
[0066] Synthesis of (R)-N-benzyl-2-N-Boc-amino-3-methoxypropionamide (compound of formula II)
[0067] In the reaction flask, dissolve 10g of (R)-2-N-Boc-amino-3-methoxypropionic acid in 80ml of dry dichloromethane, and control the temperature of the reaction solution at -15~-10°C; Add 5.6g of isobutyl chloroformate and 6.2g of N-methylmorpholine at 10~-5℃, stir for 1.5h, then add 20ml of dichloromethane dissolved in 20ml of dichloromethane dropwise at -15~-10℃ into the reaction bottle benzylamine (5.89g) solution, slowly warming up to 25-30°C within 0.5h to react for 2.0h, and HPLC was used to detect whether the reaction was complete. After the reaction is finished, wash the reaction solution with 80ml of water, 80ml of 1mol / L hydrochloric acid, 80ml of saturated sodium bicarbonate solution and 80ml of water successively, separate the lower organic phase and concentrate to dryness to obtain an oil, then add 80ml of diethyl ether: n-hexane=1 : 1 mixed solvent, cooled and stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com