Epidemic encephalitis polysaccharide-protein conjugated vaccine and preparation method thereof
A protein and polysaccharide technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of increasing the overall production cost of products, polysaccharide loss, etc., and achieve polysaccharide-protein ratio stability, The effect of low production cost and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
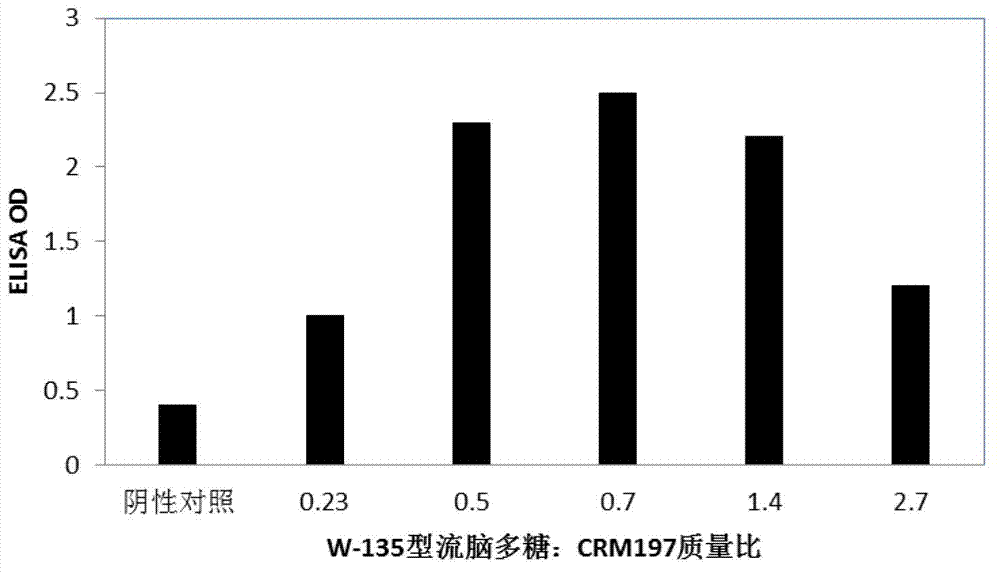
[0042] Preparation of purified meningococcal polysaccharide powder from Neisseria meningitidis serotypes W-135 and Y
[0043] Neisseria meningitidis serotypes W-135 and Y were fermented separately.
[0044] After centrifuging the 30L fermented liquid obtained respectively, the supernatant was obtained and placed in a stainless steel tank, concentrated to 3L by ultrafiltration using a 100KD membrane bag, and 300ml of cetyltrimethyl bromide with a mass concentration of 10% was added to the concentrated solution ammonium chloride (CTAB) aqueous solution, stirred evenly, and then stood still at 4°C for 2 hours. The rested solution was centrifuged to obtain a precipitate, and sterile 1M sodium chloride aqueous solution was added to 1 L of the precipitate, and the precipitate was dissolved at 40°C. Add absolute ethanol to the solution to a final volume concentration of 25%, stir evenly and stand at -20°C for 2 hours, then centrifuge to obtain the supernatant; add absolute ethanol t...
Embodiment 2
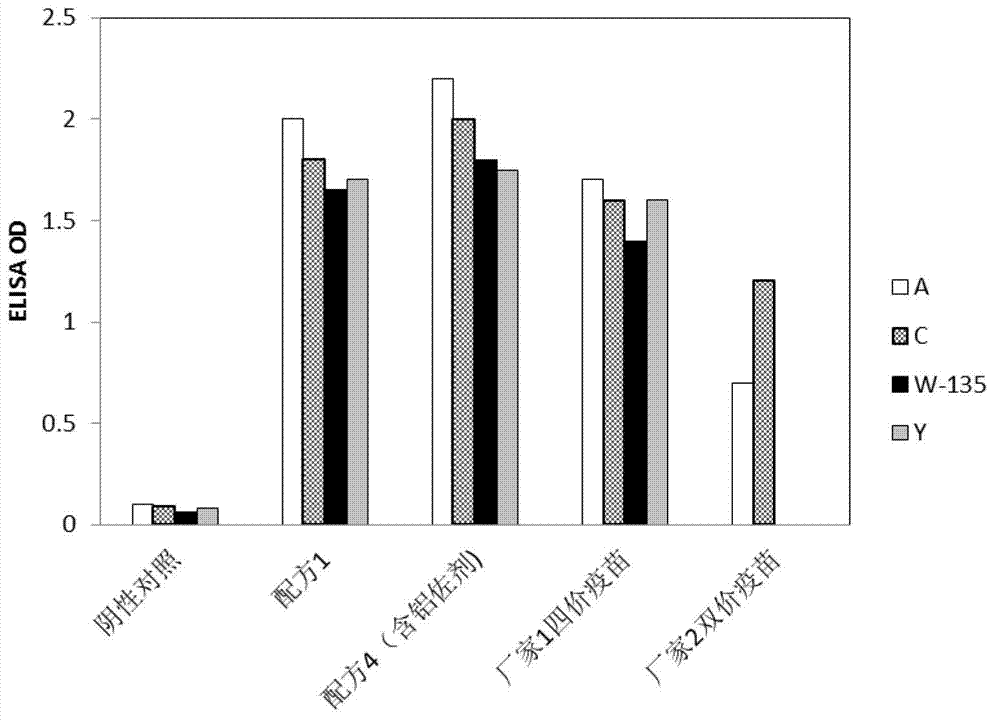
[0047] Preparation of carrier protein
[0048] Lyophilized seed cultures expressing diphtheria CRM197 protein were reconstituted and incubated for 16 hours. Transfer a portion of the culture to a 0.5-liter shaker flask containing growth medium and incubate the flask on a rotary shaker at 34.5-36.5°C for 8 hours. Transfer a portion of the culture from the flask to a 4-liter shaker flask containing growth medium Liter shaker flasks, and incubate the flasks on a rotary shaker at 34.5-36.5°C for 18 hours. The culture from this 4 liter shake flask was used to inoculate a fermenter containing 30 L of growth medium. The fermenters were incubated for 28 hours at 30-36.5°C, pH 7.4. The fermenter contents are filtered through centrifuges and depth filters into collectors.
[0049] The obtained 30L fermentation broth was concentrated to 2L with a 30KD membrane bag, and 500mM phosphate buffer (sodium salt) was added to the concentrated solution to a final concentration of 10mM. After ...
Embodiment 3
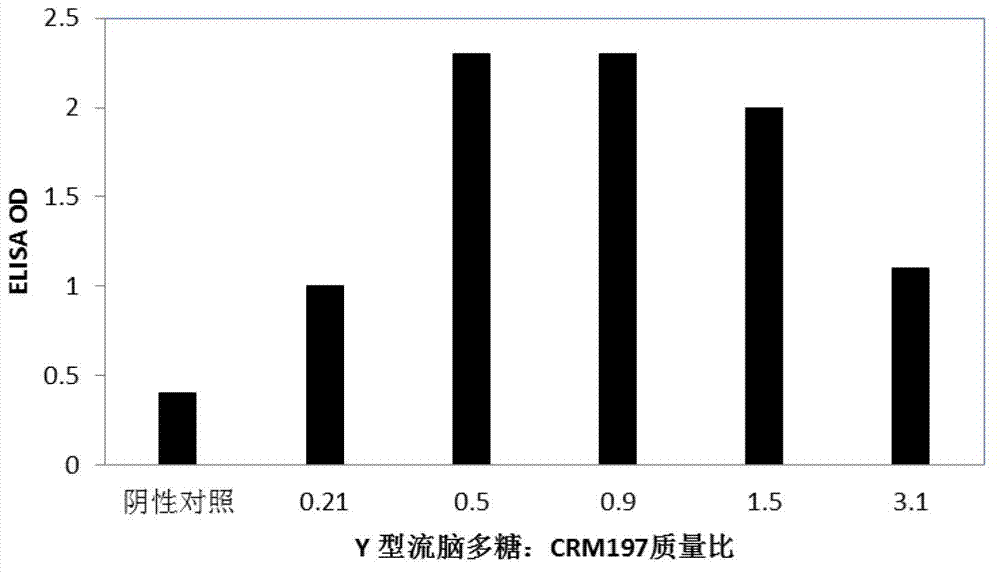
[0053] Activation of Neisseria meningitidis serotype Y polysaccharide
[0054] Dissolve 5g of the purified ECM Y-type polysaccharide in 1L of sodium acetate buffer (50mM pH5.0), and stir for 20 minutes with a magnetic stirrer with a magnet at room temperature to fully dissolve the purified ECM polysaccharide , add NaIO according to the ratio in Table 1 4 , and react respectively by the temperature and time shown in each reaction in Table 1, so that the adjacent dihydroxyl groups on the meningitis polysaccharide are oxidized into aldehyde groups.
[0055] Table 1. Activated meningitis polysaccharides with different aldehyde group introduction ratios obtained under different reaction conditions
[0056]
[0057] Prepare an ultrafiltration system equipped with a 30K MWCO filter membrane, place the reaction solution in the reflux vessel of this system, perform 10 equal-volume ultrafiltration replacements with purified water, and remove the remaining NaIO in the reaction 4 and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com