Rapid microwave preparation method of glycidyl (meth)acrylate
A technology of glycidyl ester and acrylic acid, applied in the field of microwave rapid preparation of glycidyl (meth)acrylate, can solve the problems of reducing the yield of the target product, difficult to realize industrialization, difficult to remove moisture, etc., and achieves high industrial application value and time. Short, low temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0029]Implementation Case 1 Synthesis of (meth)acrylic acid sodium salt
[0030] In this example, the mass ratio of (meth)acrylic acid to 30% metal sodium alkali solution is 1:1 to 1:5, the metal sodium alkali solution is preferably sodium hydroxide aqueous solution, and the metal sodium solution is at a speed of 1 to 10d / s, preferably 3 ~6d / s, add to (meth)acrylic acid, add 0.01%~1% polymerization inhibitor when stirring, preferably 0.01%~0.05%, prevent the polymerization of (meth)acrylic acid sodium salt, reaction temperature 0℃~80℃ , preferably 40°C-60°C, reaction time 0.5-3h, such as 1h, 2h, reaction vacuum 100-650mmHg, such as 200mmHg, 300mmHg, 400mmHg, 500mmHg, 600mmHg. After the reaction, use 5% to 30% inorganic acid, such as 10% hydrochloric acid, to adjust the pH value of the reaction solution to 8 to 10, and dry the reaction solution in vacuum at 0 to 90°C with a vacuum degree of 300 to 760mmHg to obtain a solid (meth)acrylic acid sodium salt , ground into powder, s...
Embodiment example 2
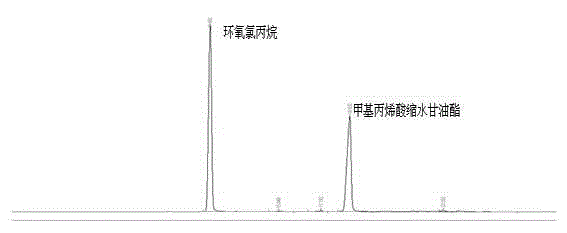
[0031] Implementation Case 2 Microwave Synthesis of Glycidyl Methacrylate
[0032] Take 50g of homemade sodium methacrylic acid, 171g of epichlorohydrin (the ratio of the two substances is 1:4), 0.6% p-hydroxyanisole and 0.6% tetrabutylammonium bromide in a 500mL microwave reaction bottle In the process, adjust the power of the microwave reactor to 500w, the temperature to 105°C, start the microwave to start timing, start sampling at the 8th minute, and stop sampling every two minutes until 20 minutes. The reaction liquid gas phase analysis shows that when the reaction time is 14 minutes, the conversion rate of the reaction reaches 95.4 %, after adding an appropriate amount of hydroquinone, the resulting product was distilled under reduced pressure to obtain a mass of glycidyl methacrylate of 58.9 g and a yield of 89.6%.
Embodiment example 3
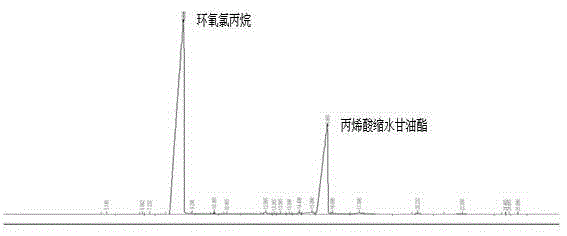
[0033] Example 3 Microwave Synthesis of Glycidyl Acrylate
[0034] Take 50g of homemade sodium acrylic acid, 171g of epichlorohydrin (the ratio of the two substances is 1:4), 0.6% p-hydroxyanisole and 0.6% tetrabutylammonium bromide in a 500mL microwave reaction bottle, Adjust the power of the microwave reactor to 500w, the temperature to 105°C, and the reaction time to 20min. Start the microwave reactor to start timing, start sampling in the 8th minute, and take samples every two minutes until 20min, gas phase analysis When the reaction time is 14min, the conversion rate of the reaction reaches 96.6%, after adding an appropriate amount of hydroquinone, the resulting The product was distilled under reduced pressure to obtain glycidyl acrylate with a mass of 58.8 g and a yield of 86.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com