Method for preparing 3-ethynyl-4-fluoroaniline
A fluoroaniline and ethynyl technology, which is applied in the field of preparation of 3-ethynyl-4-fluoroaniline, can solve the problems of large amount of catalyst used, cumbersome and difficult post-processing, unsuitable for mass preparation, etc., and achieves simplified reaction process and post-processing. The effect of treatment process, less amount of reaction solvent, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Synthesis of ((2-fluoro-5-nitrobenzene)acetylene)trimethylsilane
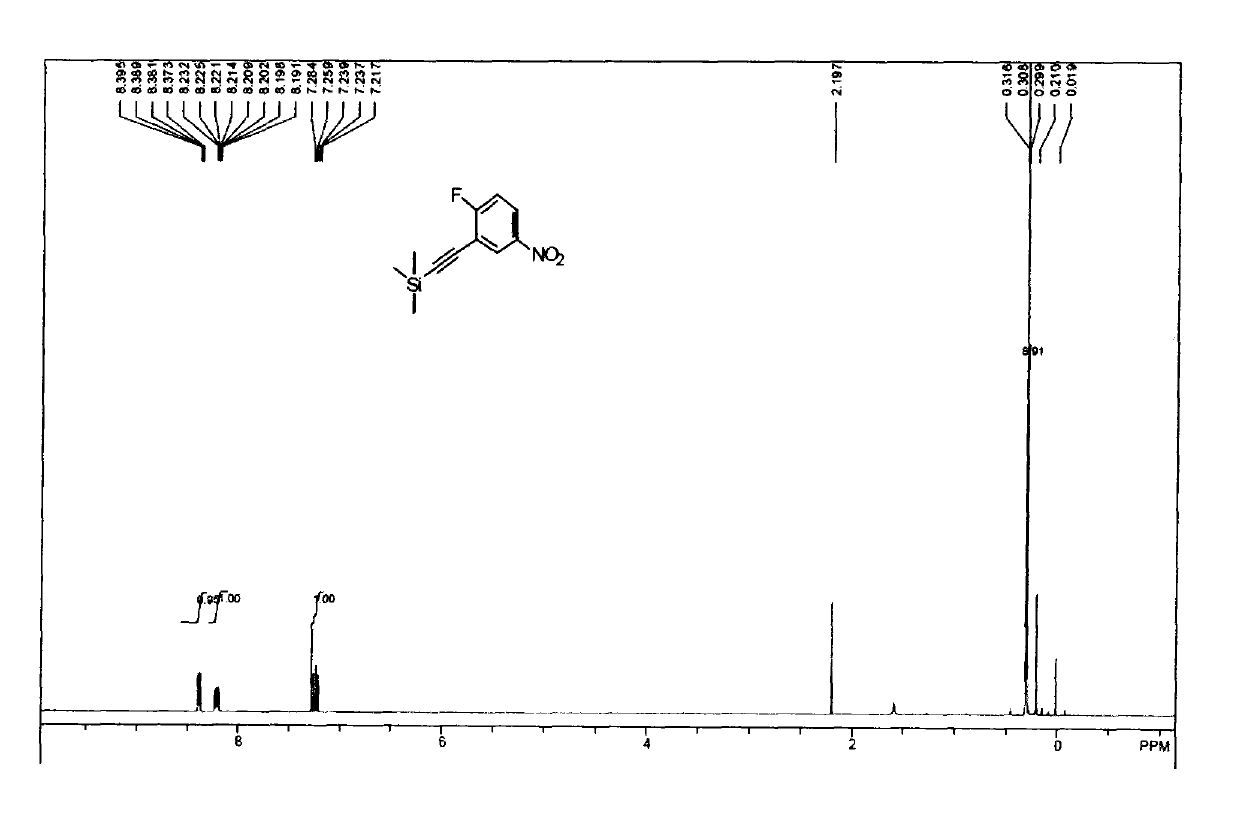
[0024] Add 3-bromo-4-fluoronitrobenzene (44g, 0.20mol) to 500ml of triethylamine, under the protection of argon, add cuprous iodide (5g, 0.02mol), tetrakistriphenylphosphorus palladium (5g , 0.004mol), and then dropwise add trimethylsilyl acetylene (29.4g, 0.30mol), 40 ~ 50 ℃ overnight. Filter, spin off the triethylamine, add 1.5L of petroleum ether, filter with a pad of silica gel, and spin dry to obtain 45 g (brown liquid) of the product (yield 95%). The NMR spectrum of the product is as figure 1 Shown: 1H-NMR (CDCl3-d6) δ8.0-8.5 (m, 2H, aromatic), 7.22 (t, 1H, J=8.2 Hz aromatic H6), 0.3 (s, 9H, SiCH3). It was confirmed that the product was ((2-fluoro-5-nitrophenyl)acetylene)trimethylsilane.
Embodiment 2
[0025] Example 2: Synthesis of 4-fluoro-3((trimethylsilane)acetylene)aniline
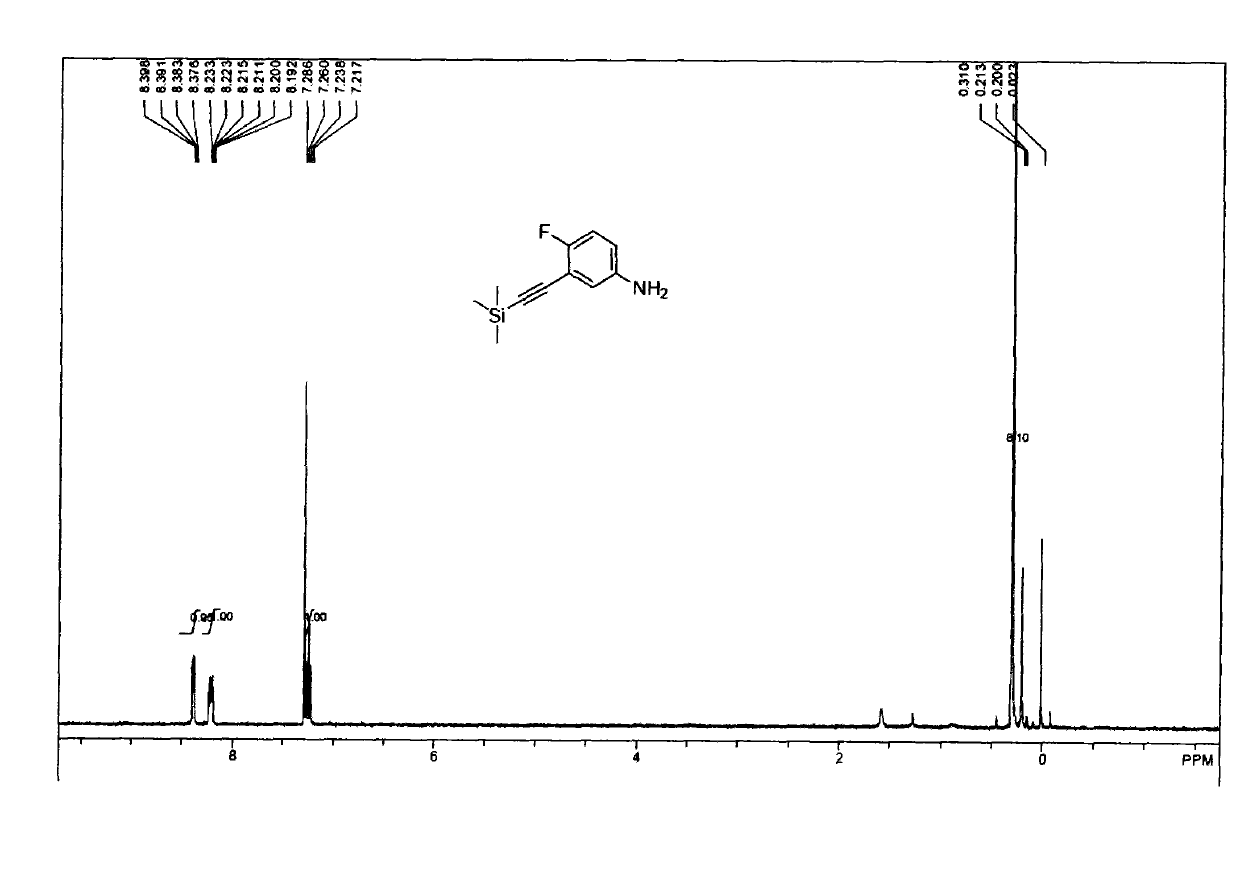
[0026] The product obtained in Example 1 (45g, 0.19mol) was dissolved in 500ml methanol, 300ml (12N) hydrochloric acid, iron powder (53g, 0.95mol) was heated to reflux for 2 hours, TLC detected, the reaction was completed, the methanol was spun off, and carbonic acid was used. Adjust the pH to alkaline with sodium hydrogen, wash the filter cake with EA (2L), add 800 ml of water to the filtrate, separate, dry and spin dry to obtain 34.2 g (yellow liquid) product, with a yield of 87%. The NMR spectrum of the product is as figure 2 Shown: 1H-NMR (400MHz, CDCl3) δ 8.39 (dd, J = 6.0, 2.9 Hz, 1H), 8.26-8.11 (m, 1H), 7.24 (t, J = 8.5 Hz, 1H), 0.31 ( s, 9H).. The product was confirmed to be 4-fluoro-3((trimethylsilane)acetylene)aniline.
Embodiment 3
[0027] Example 3: Synthesis of 3-ethynyl-4-fluoroaniline
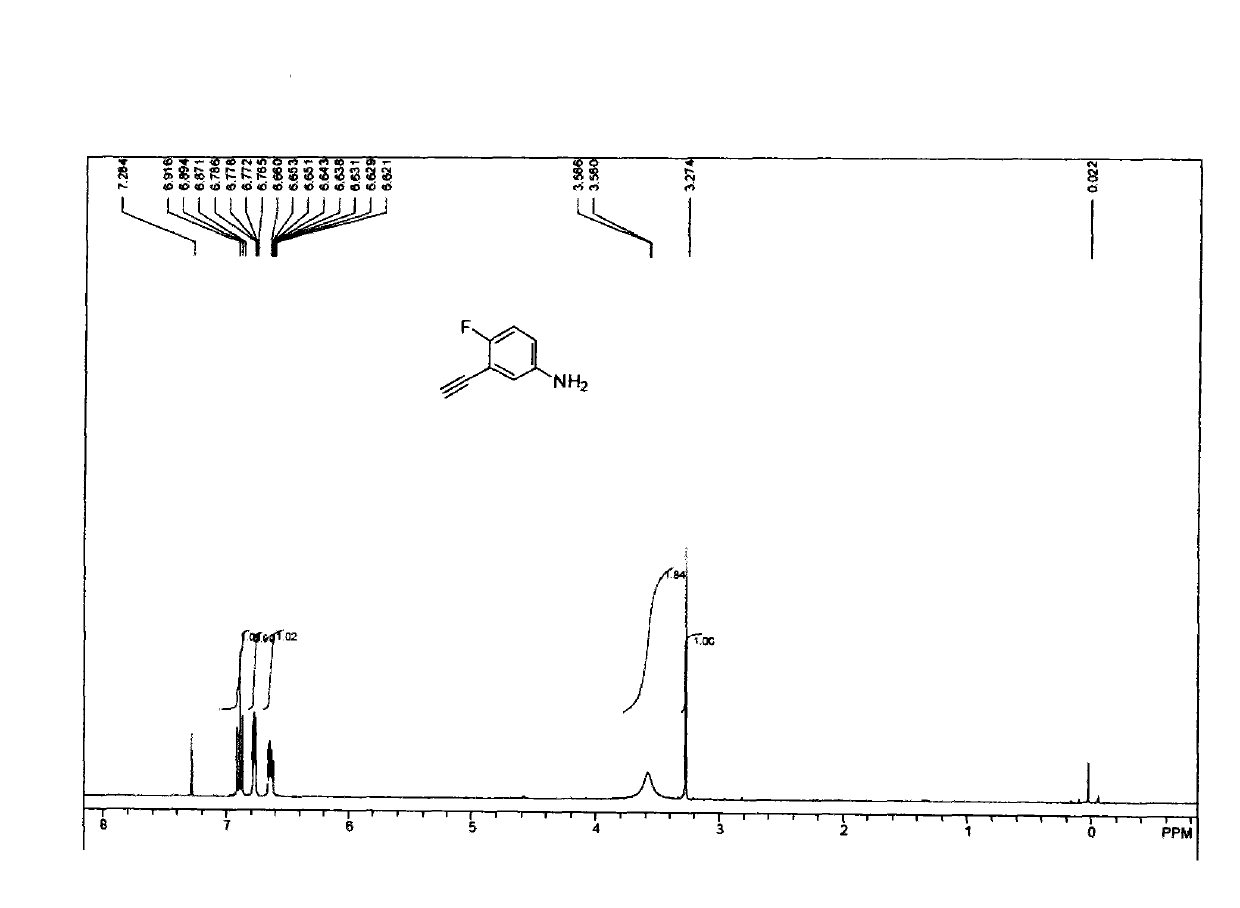
[0028] The compound (34.2g, 0.16mol) obtained in Example 2 was dissolved in 300ml methanol, potassium hydroxide (8.9g, 0.16mol) was added, stirred at room temperature for 4h, 1.5L ethyl acetate was added, and backwashed with saturated brine 2 It was dried and purified by column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain 19.6 g of (brown powder) product with a yield of 88%. The NMR spectrum of the product is as image 3 Shown as: 1H-NMR (CDCl3-d6) δ6.2-7.0 (m, 3H, aromatic), 3.6 (brs, 2H, NH2), 3.25 (s, 1H, HC≡C). The product was confirmed to be 3-ethynyl-4-fluoroaniline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com