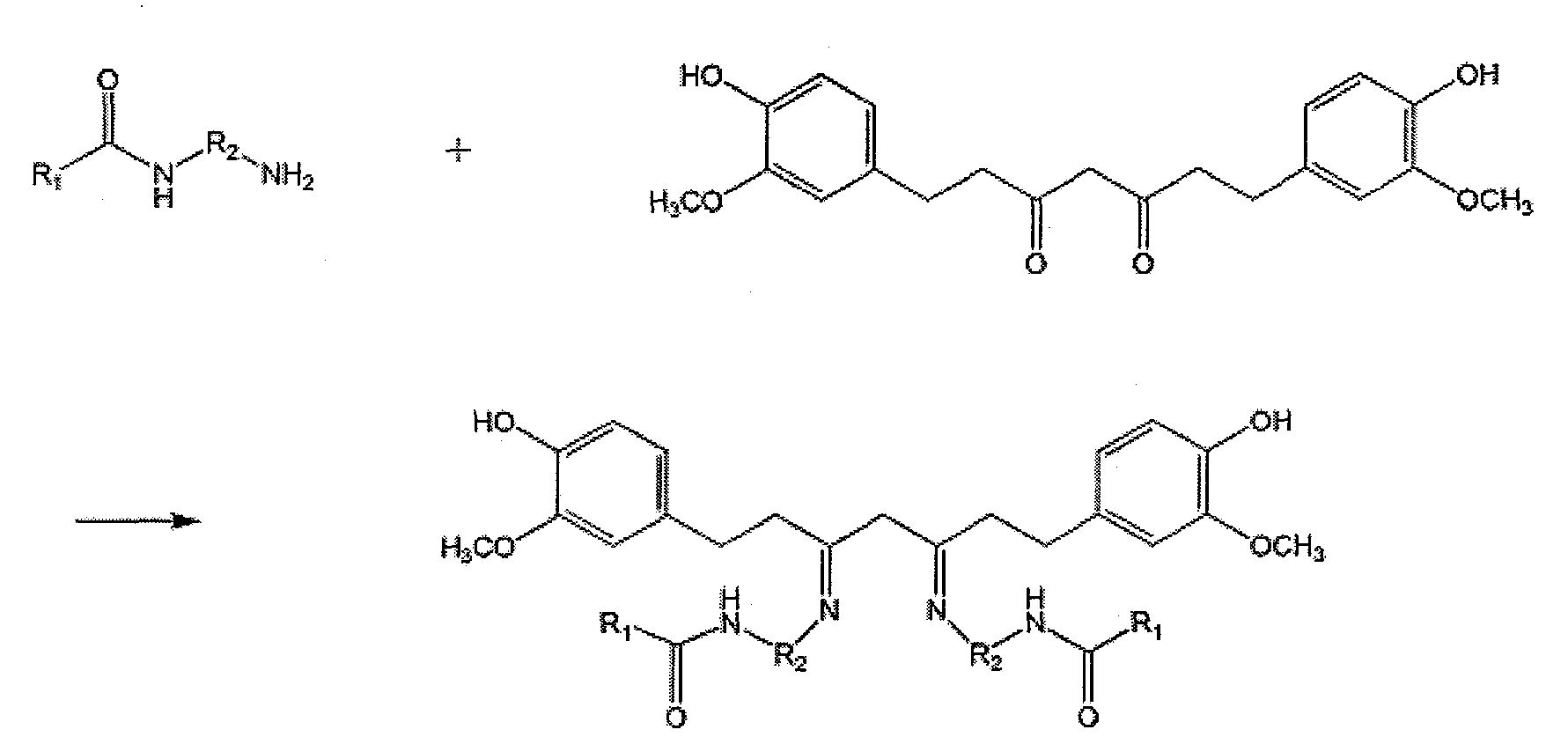
Curcumin-polysaccharide conjugate as well as preparation method and application thereof
A technology of curcumin and conjugates, which is applied in the field of biomedical materials to achieve the effects of mild synthesis conditions, high encapsulation efficiency, and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The synthesis of embodiment 1 curcumin-carboxymethyl chitosan
[0055] Add 0.1mol carboxymethyl chitosan to 10ml water, stir magnetically for 30min until completely dissolved, adjust the pH to about 6-7 with acetic acid, then add 0.5mol p-phenylenediamine, mix well and then add dropwise 0.2mol 1-ethyl -(3-Dimethylaminopropyl) carbodiimide (EDC) and 0.3mol hydroxysuccinimide (NHS), reacted at room temperature for 8 hours, dialyzed for 2 days, and freeze-dried to obtain carboxymethyl chitosan with free one-terminal amino group intermediate. Take the above intermediate and disperse it in 30ml of methanol, add 0.3mol of curcumin in methanol solution, ultrasonically reflux for 2h, the obtained crude product is washed repeatedly with absolute ethanol, anhydrous ether, and dichloromethane until the filtrate is colorless, and vacuum-dried to obtain purified curcumin-carboxymethyl chitosan conjugates.
Embodiment 2
[0056] The synthesis of embodiment 2 curcumin-heparin
[0057] Dissolve 2 mmol of heparin in formamide, add 8 mmol of ethylenediamine, stir magnetically for 2 min, then add 3 mmol of 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) and 4.5 mmol of hydroxysuccinimide Amine (NHS) was reacted at room temperature for 12 hours. After the reaction, acetone was added to precipitate the product, and the precipitate was obtained by suction filtration, redissolved in water, dialyzed for 2 days, and freeze-dried to obtain a heparin active intermediate with a free amino group at one end. Dissolve the heparin active intermediate obtained above in a methanol-water mixed solution, avoid light, and slowly add 6 mmol of curcumin in methanol solution dropwise under magnetic stirring at 50°C for 1.5 hours, and continue the reaction for 2 hours after the drop is completed Take the product by rotary evaporation to remove methanol, add a small amount of water to mix, and then filter with suction to...
Embodiment 3
[0058] The synthesis of embodiment 3 curcumin-hyaluronic acid
[0059] Dissolve 3mmol hyaluronic acid in tetrahydrofuran, add 10mmol m-phenylenediamine, stir magnetically for 5min, then add 4.5mmol 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) and 6mmol hydroxysuccinate Imide (NHS) was reacted at room temperature for 12 hours. After the reaction, acetone was added to precipitate the product, and the precipitate was obtained by suction filtration, redissolved in water, dialyzed for 2 days, and freeze-dried to obtain the active intermediate of hyaluronic acid with a free terminal amino group. Take the hyaluronic acid active intermediate obtained above and dissolve it in methanol-water mixed solution, avoid light, and slowly add 9 mmol of curcumin methanol solution dropwise under magnetic stirring at 50°C. The dropping time is 2 hours, and continue to react for 1 hour after the dropping , take the product by rotary evaporation to remove methanol, add a small amount of water ...
PUM
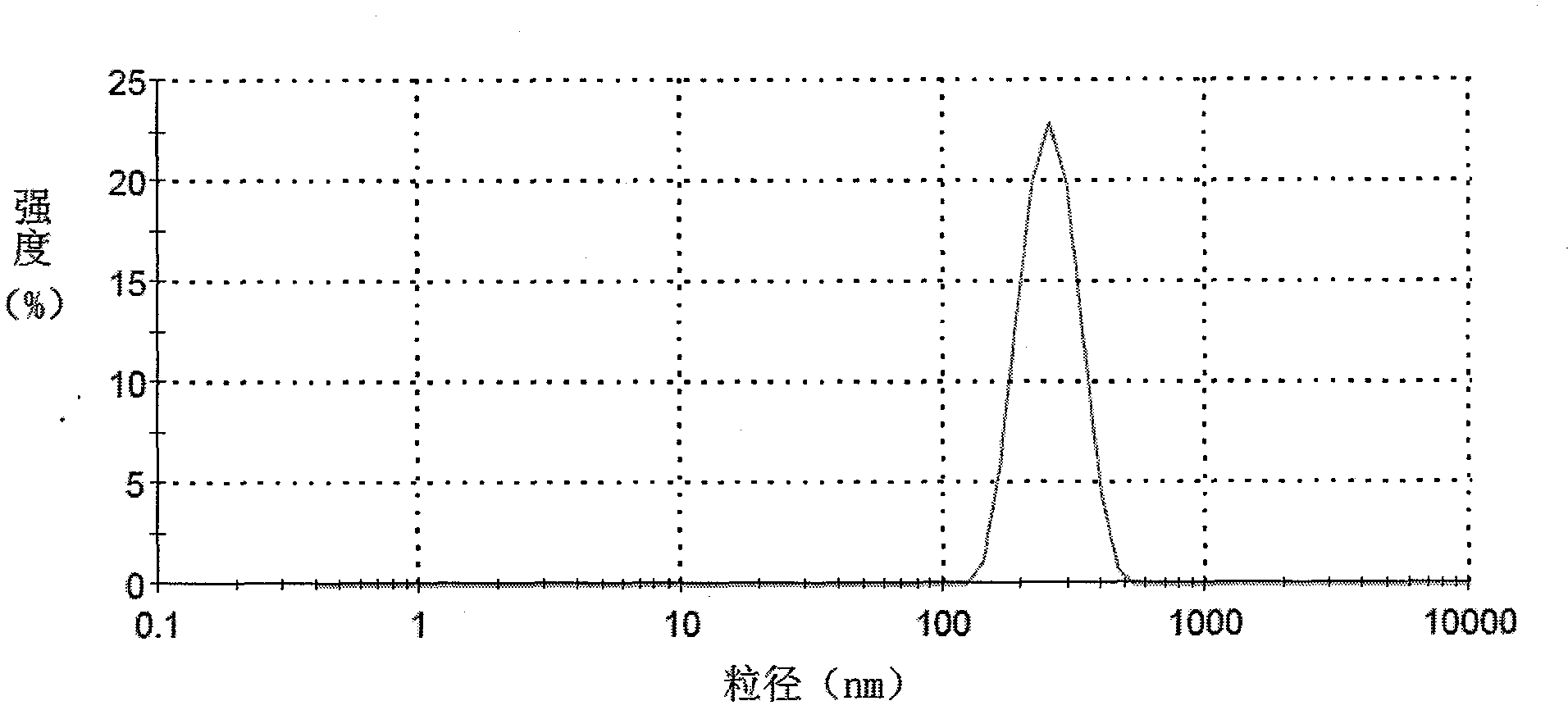
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com