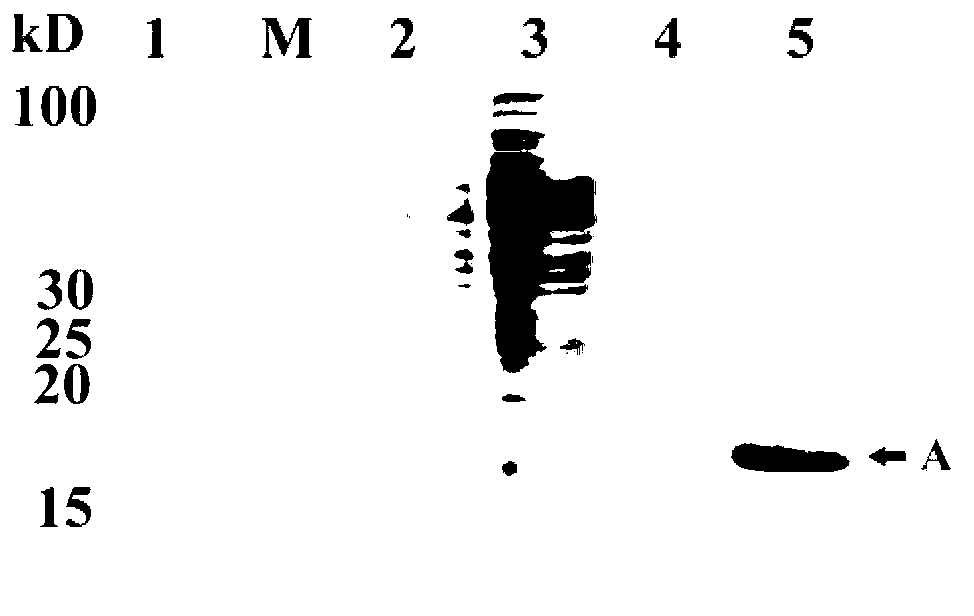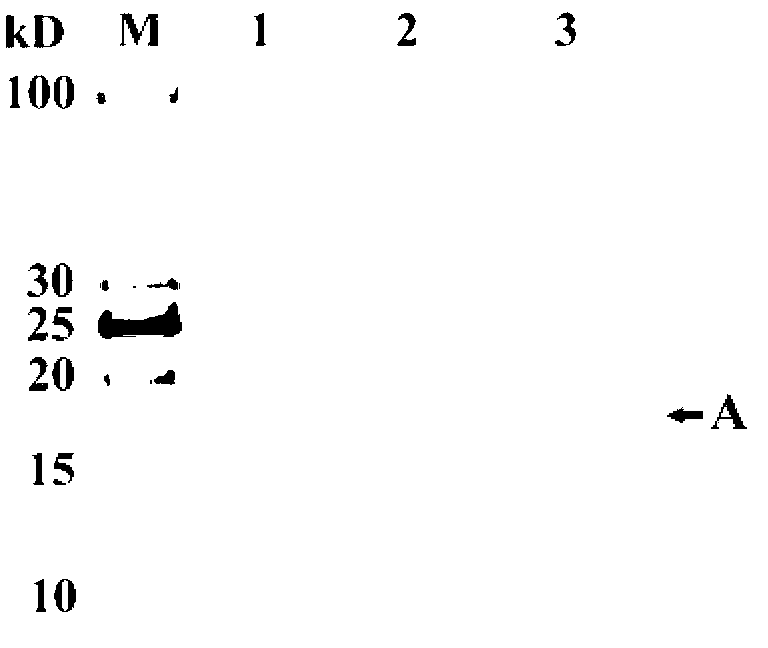Immobilization method of Thermus lipase
A lipase and lipase gene technology, applied in the field of lipase immobilization, can solve the problems of easy inactivation, difficult recovery, and limited application, and achieve easy magnetic separation and recovery, good superparamagnetism, and good operational stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Preparation of Thermus lipase (Thermus thermophilus WL)
[0040] Thermus lipase (Thermus thermophilus WL) is derived from the thermophilic bacteria (Thermus thermophilus WL) isolated from deep-sea hydrothermal vents. The Thermus lipase (Thermus thermophilus WL) gene is 486 bp in length and contains a reading frame encoding 161 amino acids (Genbank sequence registration number: AAS81965.1).
[0041] When using Escherichia coli E.Coli BL21(DE3) as the host cell, the Escherichia coli [Escherichia coli BL21(DE3)] containing the Thermus lipase gene was obtained, which was preserved in China on June 15, 2012 The General Microbiology Center of the Microbial Culture Collection Management Committee (CGMCC for short), the preservation number is CGMCC No: 1.12252.
[0042] The preparation method of described Thermus lipase is:
[0043] (1) Preparation of free Thermus lipase (Thermus thermophilus WL): Pick a single colony of Escherichia coli containing the Thermus lipa...
Embodiment 2
[0046] Example 2. Carrier preparation and Thermus lipase immobilization
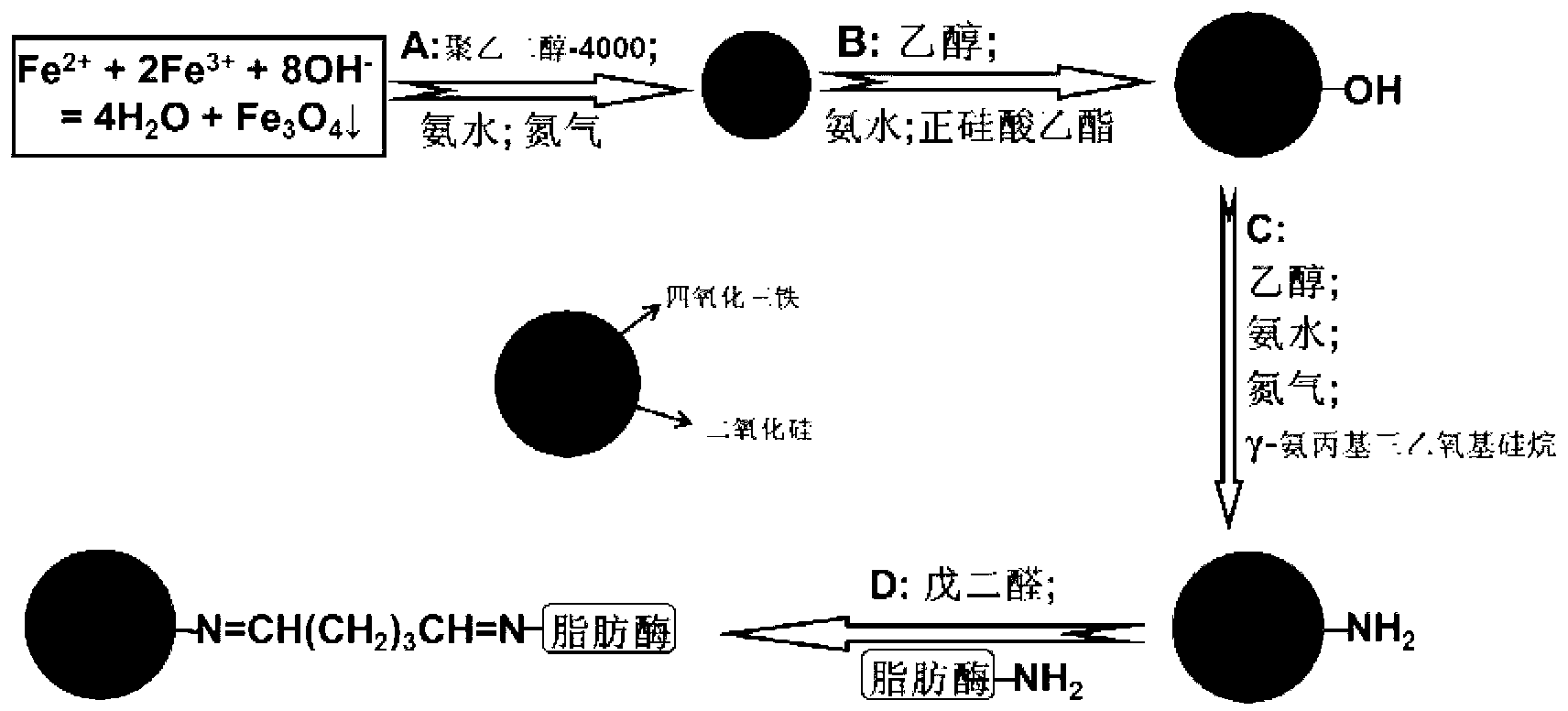
[0047] (1) High-performance magnetic nanospheres (ie Fe 3 o 4 Nanoparticles) Preparation
[0048] Fe 3 o 4 Nanoparticles are prepared by an improved chemical co-precipitation method, and the reaction formula is
[0049] Fe 2+ +2Fe 3+ +8OH-→Fe 3 o 4 +4H 2 o
[0050] Specific process: Weigh 4.054g FeCl respectively 3 ·6H 2 O and 1.988g FeCl 2 4H 2 O (considering Fe 2+ Oxidation in the preparation and subsequent process, select Fe 3+ / Fe 2+ The amount ratio of the substance is 3 / 2) placed in a 50mL beaker, add 25mL double-distilled water to dissolve and then add 90mL double-distilled water to mix to form a 115mL mixed solution, add the mixed solution to a 500mL three-necked bottle, feed nitrogen, and After stirring mechanically (1000rpm) in a water bath at 30°C for 5 minutes, add 25% NH with a mass concentration dropwise with a syringe (0.7 mm in diameter) 3 ·H 2 O10mL and 5mL of PEG (molec...
Embodiment 3
[0058] Example 3. Determination of immobilized Thermus lipase enzyme activity
[0059] The assay of lipase activity is measured with p-nitrophenol laurate (C12, p-nitrophenyl laurate) as substrate, see (Sigurgisladóttir S, Konraosdóttir M, Jónsson A, Kristjánsson J, Matthiasson E.Lipase activity of thermophilic bacteria from Icelandic hot springs.Biotechnol Lett.1993,15:361-366.), the specific operation is as follows:
[0060] 1) Preparation of C12 solution: Dissolve a certain amount of C12 in absolute ethanol to prepare a 25mM C12 ethanol solution, and store it in the dark (it is best to use it immediately).
[0061] 2) Preparation of diluted enzyme solution sample: the immobilized Thermus lipase obtained by the method in Example 2 was diluted with 50 mM Tris-HCl buffer solution (pH 8.0) to an 80 mg / L enzyme solution. Prepare calcium chloride buffer solution with 50mM Tris-HCl buffer solution (pH8.0) to make calcium chloride buffer solution with a final concentration of 40mM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene length | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com